Epidemiology of coronary heart disease and acute coronary syndrome
Introduction and definitions
Cardiovascular disease (CVD) is a group of diseases that include both the heart and blood vessels (1), thereby including coronary heart disease (CHD) and coronary artery disease (CAD), and acute coronary syndrome (ACS) among several other conditions. Although health professionals frequently use both terms CAD and ACS interchangeably, as well as CHD, they are not the same. ACS is a subcategory of CAD, whilst CHD results of CAD. On the other hand, CAD is characterized by atherosclerosis in coronary arteries and can be asymptomatic, whereas ACS almost always presents with a symptom, such as unstable angina, and is frequently associated with myocardial infarction (MI) regardless of the presence of CAD (2). Finally, CAD is usually used to refer to the pathologic process affecting the coronary arteries (usually atherosclerosis) whilst CHD includes the diagnoses of angina pectoris, MI and silent myocardial ischemia (3). In turn, CHD mortality results from CAD. For simplicity purposes, herein we will refer to CAD as CHD. Indeed, the development of novel and more sensitive immunoassays (i.e., defined as “high-sensitivity”) for measuring cardiac troponins has contributed to substantially revised this classification, wherein the spectrum of clinical conditions previously defined as “unstable angina” has now been progressively reclassified as either non-MI or MI (4).
CHD is a major cause of death and disability in developed countries (5). Although the mortality for this condition has gradually declined over the last decades in western countries, it still causes about one-third of all deaths in people older than 35 years (6-8). The Framingham Heart Study perfectly summarizes the risk factors that contribute to the development of CHD, providing critical information regarding objectives for the primary and secondary prevention of CHD.
The list of non-communicable diseases is becoming larger and more complex. Rapid globalization, urbanization, ageing of society, and an increase in chronic diseases pose new challenges to modern health care systems (9,10). CVD is preventable, but physical inactivity, nicotine abuse and bad nutrition practices (11) (lost of traditional diet habits in new-industrial cultures) are leading to an increase of prevalence in most countries (12). Further, social inequalities increase CVD-mortality (12-14) and negative lifestyle influences such as increased physical inactivity in more “obesogenic” environment (14) are reverting the improvements in CVD data that were obtained in some countries (15).
Two common measures of disease burden in a population, incidence and prevalence, are defined as follows. Incidence is the number of new cases of a disease over a period of time divided by the population at risk, whereas prevalence is the number of existing cases of a disease divided by the total population at a point in time. In this review, we summarize the incidence, prevalence, trend in mortality, and general prognosis of CHD and ACS.
Incidence, prevalence, trends in mortality, and prognosis of CHD
The 2016 Heart Disease and Stroke Statistics update of the American Heart Association (AHA) has recently reported that 15.5 million persons ≥20 years of age in the USA have CHD (16), whilst the reported prevalence increases with age for both women and men and it has been estimated that approximately every 42 seconds, an American will suffer for an MI (see Figure 1 for a summary of representative data).
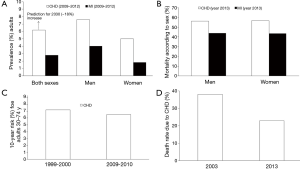
Although the absolute numbers of CVD deaths have significantly increased since the 1990, the age-standardized death rate has decreased by 22% over the same period, primarily due to a shift in age demographics and causes of death worldwide (17). In a 2009 report that used National Health and Nutrition Examination Survey (NHANES) data, MI prevalence was compared by sex in middle-aged individuals (35–54 years) during the 1988–1994 and 1999–2004 time periods (18). The prevalence of MI was higher in men compared with women in the two periods, but it tended to decline in the former over time, whilst the opposite trend was found in women. Some data based on self-reported MI and angina from health interviews, such as those from the NHANES, might underestimate the actual prevalence of advanced CHD. This is likely to be due, at least partly, by the fact that advanced occlusive CHD often exists with few symptoms or overt clinical manifestations. Silent ischemia, which accounts for 75% of all ischemic episodes (19), may be brought to light by electrocardiographic (ECG) changes on an exercise test (i.e., ST-segment depression), ambulatory 24-hour ECG recording, periodic routine ECG or cardiac troponins testing. Autopsy data have reported a reduced prevalence of anatomic CHD over time in both the general population and military personnel.
In an study which included 2,562 autopsies performed between 1979 and 1994, the prevalence of anatomically-evident CHD in subjects aged 20–59 years fell from 42% to 32% in men and from 29% to 16% in women when the periods 1979–1983 and 1990–1994 were compared (20). There was no significant change in prevalence in those aged 60 years or older. In an analysis of 3,832 autopsies performed on USA military personnel (98% male, mean age 26 years) who died during combat or due to unintentional injuries between October 2001 and August 2011, the prevalence of CHD was 8.5% (21). This represents a marked decline in the prevalence of autopsy-documented CHD compared with the rates seen during the Korean War in the 1950s (77%) and the Vietnam War in the 1960s (45%) (21).
Data from 44 years of follow-up in the original Framingham Study cohort and 20 years of surveillance of their offspring has allowed ascertainment of the incidence of initial coronary events such as MI (whether clinically recognized or not), angina pectoris, unstable angina, and sudden and non-sudden coronary deaths (22-24), reporting the following observations. First, for people aged 40 years, the lifetime risk of developing CHD was 49% in men and 32% in women whereas for those reaching age 70 years, the lifetime risk was 35% in men and 24% in women. On the other hand, for total coronary events, the incidence rose steeply with age, with women lagging behind men by 10 years whilst for the more serious manifestations of CHD, such as MI and sudden death, women lagged behind men in incidence by 20 years, but the sex ratio for incidence narrowed progressively with advancing age (7). The incidence at ages 65–94 years compared to ages 35–64 years more than doubled in men and tripled in women, respectively. Third, serious CHD manifestations (i.e., MI, sudden death) were infrequent in premenopausal women and the burden of CHD was markedly higher among postmenopausal women compared to their premenopausal age-matched referents (22). Fourth, below 65 years of age, the annual incidence of all coronary events in men (12 per 1,000) more than equaled the rate of all the other atherosclerotic cardiovascular events combined (7 per 1,000); in women, it equaled the rate of the other events (5 per 1,000). Beyond 65 years of age, CHD was still predominant. Coronary events comprised 33% to 65% of atherosclerotic cardiovascular events in men and 28% to 58% in women. Finally, the fact that angina pectoris was more frequent in men compared with women was less striking. In women aged <75 years, angina pectoris was more frequent than MI as the initial presentation of CHD (23). Furthermore, angina in women was more likely to be uncomplicated (80%), while angina in men often occurred after a MI (66%). MI predominated at virtually all ages in men in whom only 20% of events were preceded by long-standing angina; the percentage was even lower for silent or unrecognized MI (23,24).
In addition to sex, other factors may influence whether the initial presentation of CHD is an acute MI or stable angina. A case control study of adults with a first clinical presentation of CHD as either acute MI (n=916) or stable exertional angina (n=468) suggested that recent prior therapy with statins and beta blockers affect the clinical presentation (25).
The incidence of CHD has decreased over time in developed countries. Several reports have illustrated this trend. First, in an analysis from the NHANES I Epidemiologic Follow-up study, in which two cohorts of subjects were compared from 1971 to 1982 (10,869 patients) and from 1982 to 1992 (9,774 patients) (26), the incidence of CHD decreased from 133 to 114 cases per 10,000 persons per year of follow-up. An even larger decline was seen in overall CVD (from 294 to 225 cases per 10,000 persons per year). A report from the Mayo Clinic examined the incidence of CHD over time in Olmsted County (Minnesota) (27). During the interval from 1988 to 1998, there was a declining trend in the age-adjusted incidence of any new CHD (MI, sudden death, unstable angina, or angiographically-diagnosed CHD) from 57 to 50 cases per 10,000 persons [relative risk (RR) 0.91; 95% confidence interval (CI): 0.82–1.01]. On the other hand, CVD mortality has been declining recently in the USA and in regions where economies and health care systems are relatively advanced, but the experience is often quite different around the world (28). Indeed, CHD is the number one cause of death in adults from low-, middle- and high-income countries (29). At the turn of the century, it was reported that CHD mortality was expected to increase approximately 29% in women and 48% in men in developed countries between 1990 and 2020; the corresponding estimated increases in developing countries were 120% in women and 137% in men (30). On the other hand, the most dramatic increments in CHD events on a percentage basis are forecast for the Middle East and Latin America. The experience in Asia is especially important because of the large populations involved. The following are examples of differing observations made across geographic regions. In a 2014 study using World Health Organization (WHO) data from 49 countries in Europe and northern Asia, over 4 million annual deaths were due to CVD (8). In India, CHD may not be largely explained by traditional risk factors (31). In China, risk factor trends complement tracking of event rates. For example, the dramatic increase in CHD mortality in Beijing is attributable to higher cholesterol levels. The mean cholesterol level was 4.30 mmol/L (166 mg/dL) in 1984 but rose to 5.33 mmol/L (206 mg/dL) only 15 years later (32). In Latin America, declines in CVD rates have been less favorable than in the USA, with more unhealthy trends in physical activity, obesity, and smoking contributing to these differences (33). Thus, international leaders have called for action plans to avert the projected global epidemic of CHD in developing countries (34).
Given the progression of atherosclerosis over decades, patients are typically asymptomatic for years in spite of the evidence of CHD. Despite lack of symptoms, the presence and extent of non-obstructive CHD are associated with a worse prognosis compared with patients with no evidence of CHD (35,36). In a retrospective cohort study of 37,674 USA veterans (96% male) without prior CHD events who underwent coronary angiography between October 2007 and September 2012 and were followed for one year, the risk of MI increased significantly and progressively in parallel with the extent of both non-obstructive (at least one stenosis ≥20% but <70%) and obstructive CHD (at least one stenosis ≥70%) (35). Compared with patients without CHD, the risk of MI trended higher for patients with one vessel non-obstructive CHD [hazard ratio (HR) 2.0; 95% CI: 0.8–5.1] and was significantly greater for patients with non-obstructive CHD involving two (HR 4.6; 95% CI: 2.0–10.5) or three heart vessels (HR 4.5; 95% CI: 1.6–12.5). Finally, patients with non-obstructive CHD should be considered for usual secondary prevention measures (37).
What about MI?
Relatively few population-based studies have examined recent temporal trends in the incidence of MI, whether overall and by type, and more contemporary assessments of epidemiology of MI are needed to help assess the effectiveness of primary prevention and identify areas for potential improvement (38). Despite the declining incidence of CHD in the USA reported in the former section of this article, many (26,39-41), but not all (42,43), observational studies have found no reduction in the incidence of MI in a variety of time periods including 1971–1982 and 1982–1992 (26), 1975–1997 (39), 1994–1999 (40), and 1987–2006 (43). In a study of 5,832 Massachusetts patients with acute MI seen between 1975 and 1997, the incidence of MI was similar in 1975–1978 and 1997, respectively (230 per 100,000 people in both periods) (39). In a study of 2,816 cases of MI from 1976 to 2006 in Olmsted County, Minnesota, the incidence of MI decreased by 20% (186 to 141 per 100,000 population) when data of the predominant cardiac isoform of creatine kinase (CK-MB) in blood were applied (43). However, the use of the more sensitive troponin assays, which began after the year 2000 and permits the diagnosis of MI when a lower area of the myocardium is infarcted compared to CK-MB, could have potentially masked an actual reduction in MI incidence over time (44). All the patients included in the studies above, with the exception of the Olmsted County report, experienced their MI before 2000, and this potential problem does not apply to them. Approximately one quarter of MI did not meet CK-MB criteria after the introduction of troponin testing (43).
Several studies have assessed the influence of sex, race or age on the epidemiology of MI. The Atherosclerosis Risk in Communities (ARIC) study focused on the risk of CHD events among 360,000 residents aged 35–74 years in four communities: Forsythe County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland (45,46). Between 1987 and 1996, a total of 14,942 hospitalized patients with definite or probable MI were identified. The age-adjusted incidence of hospitalized MI was highest in black men and lowest among white women Although the age-adjusted incidence of first MI was relatively stable during 1987–1996 in both genders for white people (with non-significant trends to increase by 1.1% and 1.7% per year in men and women, respectively), it did increase significantly in black people (4.1% and 3.9% per year, respectively). Although the study also showed a decrease in recurrent MI in both genders (−1.9% and −2.1% per year, respectively), it was underpowered to assess differences based on race or ethnicity. Finally, case-fatality rates after MI decreased significantly during the 1987–1996 period in both genders, i.e., by −6.1% and −6.2%, respectively. The authors hypothesized that improvements in CHD outcome were mostly attributable to secondary rather than to primary prevention strategies (45-47). The Worcester Heart Attack Study has followed up a community based cohort of residents of Worcester, Massachusetts, for cardiovascular events (27,48-51). On the other hand, data from ARIC community surveillance suggests that the severity of acute MI has declined among community residents hospitalized for incident MI, which is one of the factors explaining the overall decline in CHD-related mortality (52).
In a study of patients hospitalized with MI between 1975 and 1995, the age-adjusted incidence of first MI increased initially between 1975 and 1981 (to a peak of 272 cases per 100,000), and then decreased until 1990, before having a slight increase again until 1995 (184 cases per 100,000). Among the trends observed over time, an increase in the median age of patients presenting with MI and an increase in the proportion of patients who were women, had diabetes, or had hypertension were noticed. There was no observed decrease in the crude long-term mortality rates after MI during the study period. However, when adjusted for temporal changes in CVD risk factors, there was a higher 1-year survival rate in the patients presenting in 1993 and 1995 compared with those presenting in 1975 or 1978 (RR, 1.56; 95% CI: 1.13–2.16), although the trend was not consistent during the entire period (27,48-51).
There has been a relative increase in non-ST elevation MI (NSTEMI) in relation to ST elevation MI (STEMI) with time (39,40,43). For example, a report from the National Registry of MI 1 to 5 reviewed over 2.5 million MI cases between 1990 and 2006 (40) and found that the proportion of MI due to NSTEMI increased from 19% in 1994 to 59% in 2006. This change in proportion was associated with an absolute decrease in the incidence of STEMI and either a rise (using MI defined with CK-MB or cardiac troponin criteria) or no change in the rate of NSTEMI (using MI defined with CM-MB criteria only) (43). Within a large community-based population, the incidence of MI decreased significantly after 2000, and the incidence of STEMI decreased markedly after 1999 (53). Reductions in short-term case fatality rates for MI are due, at least partly, to a decrease in the incidence of STEMI, a lower rate of death after NSTEMI (53), but also to the enhanced diagnostic effectiveness of modern cardiac troponin immunoassays for detecting minor myocardial injury.
Although many cases of MI appear to occur without warning, there is a large reservoir of detectable advanced silent CHD from which these apparently sudden events evolve. Such patients frequently have an ominous coronary risk profile and signs of pre-symptomatic CHD. Approximately 2–4% of the general population has silent coronary ischemia which despite being an asymptomatic condition can be actually detected with an exercise test or ambulatory ECG monitoring. The prevalence of this condition might be considerable higher in men with two or more major coronary risk factors (10%), and especially in patients with known CHD, e.g., 25–50% in those with stable angina detected through exercise testing or ambulatory monitoring (54).
The most specific indicator of the existence of silent myocardial ischemia in ECG recordings is a Q-wave MI (55). In a series of reports from the Framingham Heart Study, the following observations were made about silent MI. First, among patients who had suffered a new MI during routine biennial ECG, the infarct was silent in 26% of men and 34% of women, respectively (56). In men, the frequency of unrecognized MI was higher in diabetic than in non-diabetic individuals (39% vs. 18%) (57). Other large epidemiologic studies reported an overall comparable proportion of silent MI in both genders (33% of MI in men vs. 33–54% in women) (58-60). On the other hand, the short-term (<30 day) mortality after Q-wave MI has declined over the decades, but this remains to be corroborated for long-term sequelae. A study showed that substantial reductions in risk of CHD death and all-cause mortality occurred over the last decades of the previous century after Q-wave MI, coincident with improvements in post-MI therapies and in post-MI survival of individuals with depressed LV systolic function (61).
Silent MI, like clinically apparent MI, is strongly associated with age, as reflected by data of 9,141 men (48) and 13,000 women followed for 4–20 years in the Reykjavik Study (58): for instance, the incidence went from ~0 (for those aged ~40 years) to 3 per 1,000 man-year at age 60. The incidence of unrecognized MI might have been underestimated in the aforementioned biennial population-based estimates for at least two reasons: about 10% of anterior and 25% of inferior ECG MI revert to a non-diagnostic pattern within two years after the event, and some unrecognized MI precipitate sudden death before they can be discovered (e.g., type 3 MI) (62,63).
In addition to the development of new Q-waves, other ECG signs that might reflect silent CHD or an increased risk of CHD/CVD mortality are evidence of left ventricular (LV) hypertrophy (LVH), intra-ventricular conduction disturbances, and nonspecific repolarization abnormalities. The ECG evidence of LVH had a prognosis just as serious as the ECG evidence of MI in the Framingham Study (64,65). Echocardiography is more sensitive than ECG for detection of LVH. Marked deviation of the frontal T-wave axis (−180º to −15º and 105º to 180º) is indicative of a disturbance in ventricular repolarization and appears to be a predictor of CHD events and mortality in older subjects (66,67). The potential magnitude of this effect was illustrated in the Rotterdam study of 4,781 patients aged ≥55 years who were followed for a mean of four years. The presence of T-wave axis deviation was found to increase the risk of cardiac death (HR 3.9), sudden death (HR 4.4), and nonfatal cardiac events (HR 2.7) (66).
The coronary risk factor profiles of individuals with previously unrecognized MI is similar to that of patients with clinically recognized MI (55). However, two CVD risk factors, hypertension and diabetes mellitus, are associated with a higher likelihood of having unrecognized MI. Indeed, both unrecognized and recognized MI become more incident with severe hypertension, but the proportion of unrecognized MI is considerably higher in hypertensive people compared to their normotensive peers (68). This trend persisted even when excluding from the analyses patients with diabetes or LVH, or those receiving antihypertensive therapy. On the other hand, diabetes was confirmed by the Framingham Study as a risk factor for silent infarction in men but not in women (57). In men with diabetes, the fraction of MI that were unrecognized was more than two times higher than in those without (39% vs. 18%); in comparison, women with diabetes in this study (36) as well as in the Heart and Estrogen/progestin Replacement Study (HERS), were less likely to have unrecognized MI (57,69).
The long-term outcomes of individuals with established CHD have been evaluated with special attention to sex differences. In a survey from Rochester (MN, USA) including patients seen between 1960 and 1979, women with angina pectoris as an initial diagnosis had a longer survival and lower risk of subsequent MI or cardiac death than age-matched men with the same presentation (70). This difference did not apply to presentations with MI or sudden cardiac arrest. A later analysis from Finland suggested that the prognosis may not be different in women (71). Data were collected on nearly 120,000 patients aged 45–89 years who had new onset of “nitrate angina” or “test-positive angina”. Nitrate angina was associated with a similar increase in coronary mortality at four years compared to the general population in both women and men at every age group. However, among patients aged <75 years and with test-positive angina, the coronary mortality ratio was higher in women. Among elderly women and men, exertional chest pain has been linked with the same increase in RR of coronary but not of non-coronary mortality (72). The incidence/prevalence of MI increases progressively in older women, especially after the age of 45 (73). Women with a first symptomatic MI are usually ~6–10 years older than men (by 6 to 10 years) (74) as well as more likely to have a history of diabetes, hypertension, hyperlipidemia, heart failure, and an unstable angina pattern (74,75).
Several studies have specifically evaluated the outcomes in women with ACS and no ST elevation [unstable angina or NSTEMI (non-Q wave)]. Despite more comorbidities, these women have a similar (76) or better outcome than men (77-79). In the Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb study, which evaluated 12,142 patients with ACS, there was no outcome difference between genders when considering NSTEMI patients, while women with unstable angina had a lower rate of death or re-infarction [odds ratio (OR) 0.65] (78). Similar findings were reported in another study of 2,271 patients who were followed for six years after they presented to the emergency department for the first time with unstable angina (79). After multivariate analysis, women had a trend toward a lower risk of death (RR, 0.81) and a significantly reduced risk for a cardiac event (RR, 0.83).
CHD: cause of death
CHD is the leading cause of death in adults in the U.S., accounting for ~one-third of all deaths in subjects over age 35 (7). The 2016 Heart Disease and Stroke Statistics update of the AHA reported that overall death rate from CHD was 102.6 per 100,000 (16). Moreover, from 2003 to 2013, the annual death rate attributable to CHD declined 38.0% and the actual number of deaths declined 22.9% (16).
Although the trend has tended to reach a plateau since 1990, the overall mortality rates for CVD and CHD have fallen in most developed countries (by 24–50%) since the 1975 (7,26,46,50,80-87). The causes for reduction in CHD mortality were evaluated in adults aged 25–84 years in the USA during the 1980–2000 period (85), and the following main findings arose. First, approximately one-half of this effect was accounted for factors like improvements in therapy, including secondary preventive measures after MI or revascularization, initial treatments for ACS, therapy for heart failure, and revascularization for chronic angina accounted for approximately one-half of the decline in CHD mortality. The other half of this effect was due to changes in risk factors, including reductions in total cholesterol (24%), systolic blood pressure (20%), smoking (12%), and physical inactivity (5%). However, the aforementioned reductions were partly offset by increases in body mass index and in the prevalence of diabetes (88). Similar trends toward an outcome improvement in developed countries have been described in an analysis of death certificates from the WHO database (89). The following findings were noted for the 1965–1969 and 1995–1997 periods. First, in the USA, CHD mortality fell by 63% in men (331 to 121 per 100,000) and by 60% in women (166 to 67 per 100,000). In the European Union, CHD mortality fell by 32% in men (146 to 100 per 100,000) and by 30% in women (64 to 45 per 100,000). There was some variability in Eastern Europe, with some countries showing an increase in CHD mortality in the early 1990s followed by a subsequent decline (Poland and the Czech Republic). The highest CHD mortality was found in the Russian Federation, i.e., 330 and 154 per 100,000 in men and women, respectively, for the 1995–1998 period, with these values being still similar to those for 1985–1989. In Japan, CHD mortality was much lower than in the USA or Europe, and fell by 29% in men (50 to 36 per 100,000) and by 36% in women (28 to 18 per 100,000), respectively. Unlike the above data, mortality from CHD is expected to increase in developing countries (China, India, sub-Saharan Africa, Latin America, and the Middle East), from an estimated 9 million in 1990 to a projected 19 million by 2020 (90,91). This phenomenon is to be expected as a consequence of social and economic changes in these countries, resulting in higher life expectancy, Westernized diets, physical inactivity, increases in cigarette smoking and environmental pollution (32).
Sudden cardiac death (SCD)
There is a strong relationship between SCD and CHD (92). Clinical and post-mortem studies as well as data from death certificates revealed that 62–85% of patients who suffer out-of-hospital SCD have evidence of prior CHD, 10% have other structural cardiac abnormalities, and 5% have no structural cardiac abnormality (93,94). A surveillance study of SCD from Ireland concluded that the majority of cases occurred in home and that successful resuscitation of SCD was especially associated with ventricular fibrillation as presenting rhythm (95). A recent study reported evidence for the occurrence of active Coxsackie B viral infections in people who died of MI compared to controls, and the authors suggested that an underlying mechanism might be disruption of dystrophin in endomyocardial tissue (96).
SCD is the initial clinical coronary event in 15% of patients with CHD (76). In addition, SCD is the most frequent type of death in patients with CHD, accounting for 30% to 50% of events (97,98). The incidence of SCD after acute MI is the same with STEMI and NSTEMI (99), as well as with symptomatic and silent MI (100). Among patients who have had an MI and are followed for about four years, approximately one-half of sudden deaths occur in the first year and one-quarter in the first three months (101,102). The risk is especially high in patients with a LV ejection fraction ≤35%. On the other hand, although the risk of SCD is the highest in patients with a history of prior resuscitated SCD, MI or heart failure, approximately 80% of SCD events occur in asymptomatic patients with no such history (103). Among patients with CHD, the sudden death risk in women is one-half that in men, and in asymptomatic persons the risk for SCD is generally proportional to risk of CVD, with the incidence lags behind men by more than 10 years (98). In both sexes, MI doubles the risk of SCD compared with angina (98,100). In the Oregon Sudden Unexpected Death Study (Ore-SUDS) of over 1,500 cases of sudden cardiac arrest, the following main findings were noted (104). First, there was no significant gender effect in the prevalence of obesity, dyslipidemia, LVH, or history of MI. Second, women had a significantly less risk of having severe LV dysfunction (OR 0.51) or CHD (OR 0.34). The outcome of women who experienced an episode of out-of-hospital SCD was examined in a retrospective cohort study of 9,651 men and women (105). Women were less likely than men to have ventricular fibrillation as an initial rhythm dysfunction (25% vs. 43%) and were more likely to have pulseless electrical activity/asystole (73% vs. 55%). After adjusting for presence of ventricular fibrillation and other factors, women had a similar rate of survival to hospital discharge (29% vs. 28%).
Conclusions and perspectives
Although CHD mortality rates have declined over the past four decades in western countries, this condition remains responsible for ~one-third of all deaths in individuals over age 35. Nearly one-half of all middle-aged men and one-third of middle-aged women in the USA will develop some manifestation of CHD. The 2016 Heart Disease and Stroke Statistics update of the AHA reported that 15.5 million people in the USA. have CHD. The reported prevalence increases with age for both women and men. For those US people, the lifetime risk of developing CHD with ≥2 major risk factors is 37.5% for men and 18.3% for women. CVD disease mortality has been declining in the USA and in regions where economies and health care systems are relatively advanced, but the experience is often quite different around the globe.
Acknowledgements
Funding: The research of AL is funded by the Fondo de Investigaciones Sanitarias (FIS, grant # PI12/00914 and) and co-financed by Fondos FEDER.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: World Health Organization, 2011.
- Lippi G, Sanchis-Gomar F, Cervellin G. Chest pain, dyspnea and other symptoms in patients with type 1 and 2 myocardial infarction. A literature review. Int J Cardiol 2016;215:20-2. [Crossref] [PubMed]
- Cervellin G, Lippi G. Of MIs and men--a historical perspective on the diagnostics of acute myocardial infarction. Semin Thromb Hemost 2014;40:535-43. [Crossref] [PubMed]
- Cervellin G, Mattiuzzi C, Bovo C, et al. Diagnostic algorithms for acute coronary syndrome-is one better than another? Ann Transl Med 2016;4:193. [Crossref] [PubMed]
- Roger VL. Epidemiology of myocardial infarction. Med Clin North Am 2007;91:537-52. ix. [Crossref] [PubMed]
- Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117:e25-146. [Crossref] [PubMed]
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010;121:948-54. [Crossref] [PubMed]
- Nichols M, Townsend N, Scarborough P, et al. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J 2014;35:2929. [Crossref] [PubMed]
- Horton R. Offline: Chronic diseases—the social justice issue of our time. Lancet 2015;386:2378. [Crossref]
- Danaei G, Singh GM, Paciorek CJ, et al. The Global Cardiovascular Risk Transition: Associations of Four Metabolic Risk Factors with National Income, Urbanization, and Western Diet in 1980 and 2008. Circulation 2013;127:1493-502. [Crossref] [PubMed]
- Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 2009;6:e1000058. [Crossref] [PubMed]
- World Health Organisation. World Health Report. 2013. Available online: http://www.who.int/whr/2013/report/en/
- Wilkinson RG, Pickett KE. Income inequality and population health: a review and explanation of the evidence. Soc Sci Med 2006;62:1768-84. [Crossref] [PubMed]
- Leischik R, Dworrak B, Strauss M, et al. Plasticity of Health. German Journal of Medicine 2016;1:1-17.
- Laatikainen T, Critchley J, Vartiainen E, et al. Explaining the decline in coronary heart disease mortality in Finland between 1982 and 1997. Am J Epidemiol 2005;162:764-73. [Crossref] [PubMed]
- Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 2016;133:447-54. [Crossref] [PubMed]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117-71. [Crossref] [PubMed]
- Towfighi A, Zheng L, Ovbiagele B. Sex-specific trends in midlife coronary heart disease risk and prevalence. Arch Intern Med 2009;169:1762-6. [Crossref] [PubMed]
- Deedwania PC, Carbajal EV. Silent myocardial ischemia. A clinical perspective. Arch Intern Med 1991;151:2373-82. [Crossref] [PubMed]
- Roger VL, Weston SA, Killian JM, et al. Time trends in the prevalence of atherosclerosis: a population-based autopsy study. Am J Med 2001;110:267-73. [Crossref] [PubMed]
- Webber BJ, Seguin PG, Burnett DG, et al. Prevalence of and risk factors for autopsy-determined atherosclerosis among US service members, 2001-2011. JAMA 2012;308:2577-83. [Crossref] [PubMed]
- Gordon T, Kannel WB, Hjortland MC, et al. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med 1978;89:157-61. [Crossref] [PubMed]
- Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J 1986;111:383-90. [Crossref] [PubMed]
- Kannel WB. Prevalence and clinical aspects of unrecognized myocardial infarction and sudden unexpected death. Circulation 1987;75:II4-5. [PubMed]
- Go AS, Iribarren C, Chandra M, et al. Statin and beta-blocker therapy and the initial presentation of coronary heart disease. Ann Intern Med 2006;144:229-38. [Crossref] [PubMed]
- Ergin A, Muntner P, Sherwin R, et al. Secular trends in cardiovascular disease mortality, incidence, and case fatality rates in adults in the United States. Am J Med 2004;117:219-27. [Crossref] [PubMed]
- Arciero TJ, Jacobsen SJ, Reeder GS, et al. Temporal trends in the incidence of coronary disease. Am J Med 2004;117:228-33. [Crossref] [PubMed]
- Yusuf S, Reddy S, Ounpuu S, et al. Global burden of cardiovascular diseases: Part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation 2001;104:2855-64. [Crossref] [PubMed]
- Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747-57. [Crossref] [PubMed]
- Yusuf S, Reddy S, Ounpuu S, et al. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001;104:2746-53. [Crossref] [PubMed]
- Goyal A, Yusuf S. The burden of cardiovascular disease in the Indian subcontinent. Indian J Med Res 2006;124:235-44. [PubMed]
- Critchley J, Liu J, Zhao D, et al. Explaining the increase in coronary heart disease mortality in Beijing between 1984 and 1999. Circulation 2004;110:1236-44. [Crossref] [PubMed]
- Rodríguez T, Malvezzi M, Chatenoud L, et al. Trends in mortality from coronary heart and cerebrovascular diseases in the Americas: 1970-2000. Heart 2006;92:453-60. [Crossref] [PubMed]
- Beaglehole R, Reddy S, Leeder SR. Poverty and human development: the global implications of cardiovascular disease. Circulation 2007;116:1871-3. [Crossref] [PubMed]
- Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 2014;312:1754-63. [Crossref] [PubMed]
- Chow BJ, Small G, Yam Y, et al. Incremental prognostic value of cardiac computed tomography in coronary artery disease using CONFIRM: COroNary computed tomography angiography evaluation for clinical outcomes: an InteRnational Multicenter registry. Circ Cardiovasc Imaging 2011;4:463-72. [Crossref] [PubMed]
- Fleg JL, Forman DE, Berra K, et al. Secondary prevention of atherosclerotic cardiovascular disease in older adults: a scientific statement from the American Heart Association. Circulation 2013;128:2422-46. [Crossref] [PubMed]
- Yeh RW, Go AS. Rethinking the epidemiology of acute myocardial infarction: challenges and opportunities. Arch Intern Med 2010;170:759-64. [Crossref] [PubMed]
- Furman MI, Dauerman HL, Goldberg RJ, et al. Twenty-two year (1975 to 1997) trends in the incidence, in-hospital and long-term case fatality rates from initial Q-wave and non-Q-wave myocardial infarction: a multi-hospital, community-wide perspective. J Am Coll Cardiol 2001;37:1571-80. [Crossref] [PubMed]
- Rogers WJ, Frederick PD, Stoehr E, et al. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J 2008;156:1026-34. [Crossref] [PubMed]
- Chen J, Normand SL, Wang Y, et al. Recent declines in hospitalizations for acute myocardial infarction for Medicare fee-for-service beneficiaries: progress and continuing challenges. Circulation 2010;121:1322-8. [Crossref] [PubMed]
- Hardoon SL, Whincup PH, Lennon LT, et al. How much of the recent decline in the incidence of myocardial infarction in British men can be explained by changes in cardiovascular risk factors? Evidence from a prospective population-based study. Circulation 2008;117:598-604. [Crossref] [PubMed]
- Roger VL, Weston SA, Gerber Y, et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation 2010;121:863-9. [Crossref] [PubMed]
- Parikh NI, Gona P, Larson MG, et al. Long-term trends in myocardial infarction incidence and case fatality in the National Heart, Lung, and Blood Institute's Framingham Heart study. Circulation 2009;119:1203-10. [Crossref] [PubMed]
- Watkins S, Thiemann D, Coresh J, et al. Fourteen-year (1987 to 2000) trends in the attack rates of, therapy for, and mortality from non-ST-elevation acute coronary syndromes in four United States communities. Am J Cardiol 2005;96:1349-55. [Crossref] [PubMed]
- Rosamond WD, Chambless LE, Folsom AR, et al. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med 1998;339:861-7. [Crossref] [PubMed]
- Goldberg RJ, Yarzebski J, Lessard D, et al. A two-decades (1975 to 1995) long experience in the incidence, in-hospital and long-term case-fatality rates of acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol 1999;33:1533-9. [Crossref] [PubMed]
- Goldberg RJ, Glatfelter K, Burbank-Schmidt E, et al. Trends in community mortality due to coronary heart disease. Am Heart J 2006;151:501-7. [Crossref] [PubMed]
- Goldberg RJ, McCormick D, Gurwitz JH, et al. Age-related trends in short- and long-term survival after acute myocardial infarction: a 20-year population-based perspective (1975-1995). Am J Cardiol 1998;82:1311-7. [Crossref] [PubMed]
- McGovern PG, Jacobs DR Jr, Shahar E, et al. Trends in acute coronary heart disease mortality, morbidity, and medical care from 1985 through 1997: the Minnesota heart survey. Circulation 2001;104:19-24. [Crossref] [PubMed]
- Roger VL, Jacobsen SJ, Weston SA, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med 2002;136:341-8. [Crossref] [PubMed]
- Myerson M, Coady S, Taylor H, et al. Declining severity of myocardial infarction from 1987 to 2002: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2009;119:503-14. [Crossref] [PubMed]
- Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155-65. [Crossref] [PubMed]
- American Diabetes Association. 8. Cardiovascular Disease and Risk Management. Diabetes Care 2016;39 Suppl 1:S60-71. [Crossref] [PubMed]
- Sheifer SE, Manolio TA, Gersh BJ. Unrecognized myocardial infarction. Ann Intern Med 2001;135:801-11. [Crossref] [PubMed]
- Kannel WB, Cupples LA, Gagnon DR. Incidence, precursors and prognosis of unrecognized myocardial infarction. Adv Cardiol 1990;37:202-14. [Crossref] [PubMed]
- Kannel WB. Lipids, diabetes, and coronary heart disease: insights from the Framingham Study. Am Heart J 1985;110:1100-7. [Crossref] [PubMed]
- Sigurdsson E, Thorgeirsson G, Sigvaldason H, et al. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris. The Reykjavik Study. Ann Intern Med 1995;122:96-102. [Crossref] [PubMed]
- Jónsdóttir LS, Sigfusson N, Sigvaldason H, et al. Incidence and prevalence of recognised and unrecognised myocardial infarction in women. The Reykjavik Study. Eur Heart J 1998;19:1011-8. [Crossref] [PubMed]
- de Torbal A, Boersma E, Kors JA, et al. Incidence of recognized and unrecognized myocardial infarction in men and women aged 55 and older: the Rotterdam Study. Eur Heart J 2006;27:729-36. [Crossref] [PubMed]
- Guidry UC, Evans JC, Larson MG, et al. Temporal trends in event rates after Q-wave myocardial infarction: the Framingham Heart Study. Circulation 1999;100:2054-9. [Crossref] [PubMed]
- Davidoff R, Goldman AP, Diamond TH, et al. The natural history of the Q wave in inferoposterior myocardial infarction. S Afr Med J 1982;61:611-2. [PubMed]
- Richter A, Herlitz J, Hjalmarson A. QRS complex recovery during one year after acute myocardial infarction. Clin Cardiol 1987;10:16-20. [Crossref] [PubMed]
- Kannel WB, Abbott RD. A prognostic comparison of asymptomatic left ventricular hypertrophy and unrecognized myocardial infarction: the Framingham Study. Am Heart J 1986;111:391-7. [Crossref] [PubMed]
- Levy D, Salomon M, D'Agostino RB, et al. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation 1994;90:1786-93. [Crossref] [PubMed]
- Kors JA, de Bruyne MC, Hoes AW, et al. T axis as an indicator of risk of cardiac events in elderly people. Lancet 1998;352:601-5. [Crossref] [PubMed]
- Rautaharju PM, Nelson JC, Kronmal RA, et al. Usefulness of T-axis deviation as an independent risk indicator for incident cardiac events in older men and women free from coronary heart disease (the Cardiovascular Health Study). Am J Cardiol 2001;88:118-23. [Crossref] [PubMed]
- Kannel WB, Dannenberg AL, Abbott RD. Unrecognized myocardial infarction and hypertension: the Framingham Study. Am Heart J 1985;109:581-5. [Crossref] [PubMed]
- Shlipak MG, Elmouchi DA, Herrington DM, et al. The incidence of unrecognized myocardial infarction in women with coronary heart disease. Ann Intern Med 2001;134:1043-7. [Crossref] [PubMed]
- Orencia A, Bailey K, Yawn BP, et al. Effect of gender on long-term outcome of angina pectoris and myocardial infarction/sudden unexpected death. JAMA 1993;269:2392-7. [Crossref] [PubMed]
- Hemingway H, McCallum A, Shipley M, et al. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA 2006;295:1404-11. [Crossref] [PubMed]
- LaCroix AZ, Guralnik JM, Curb JD, et al. Chest pain and coronary heart disease mortality among older men and women in three communities. Circulation 1990;81:437-46. [Crossref] [PubMed]
- Maddox TM, Reid KJ, Spertus JA, et al. Angina at 1 year after myocardial infarction: prevalence and associated findings. Arch Intern Med 2008;168:1310-6. [Crossref] [PubMed]
- White HD, Barbash GI, Modan M, et al. After correcting for worse baseline characteristics, women treated with thrombolytic therapy for acute myocardial infarction have the same mortality and morbidity as men except for a higher incidence of hemorrhagic stroke. The Investigators of the International Tissue Plasminogen Activator/Streptokinase Mortality Study. Circulation 1993;88:2097-103. [Crossref] [PubMed]
- Maynard C, Litwin PE, Martin JS, et al. Gender differences in the treatment and outcome of acute myocardial infarction. Results from the Myocardial Infarction Triage and Intervention Registry. Arch Intern Med 1992;152:972-6. [Crossref] [PubMed]
- Hochman JS, McCabe CH, Stone PH, et al. Outcome and profile of women and men presenting with acute coronary syndromes: a report from TIMI IIIB. TIMI Investigators. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol 1997;30:141-8. [Crossref] [PubMed]
- Chang WC, Kaul P, Westerhout CM, et al. Impact of sex on long-term mortality from acute myocardial infarction vs unstable angina. Arch Intern Med 2003;163:2476-84. [Crossref] [PubMed]
- Hochman JS, Tamis JE, Thompson TD, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med 1999;341:226-32. [Crossref] [PubMed]
- Roger VL, Farkouh ME, Weston SA, et al. Sex differences in evaluation and outcome of unstable angina. JAMA 2000;283:646-52. [Crossref] [PubMed]
- Kuulasmaa K, Tunstall-Pedoe H, Dobson A, et al. Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. Lancet 2000;355:675-87. [Crossref] [PubMed]
- McGovern PG, Pankow JS, Shahar E, et al. Recent trends in acute coronary heart disease--mortality, morbidity, medical care, and risk factors. The Minnesota Heart Survey Investigators. N Engl J Med 1996;334:884-90. [Crossref] [PubMed]
- Capewell S, Morrison CE, McMurray JJ. Contribution of modern cardiovascular treatment and risk factor changes to the decline in coronary heart disease mortality in Scotland between 1975 and 1994. Heart 1999;81:380-6. [Crossref] [PubMed]
- Capewell S, Beaglehole R, Seddon M, et al. Explanation for the decline in coronary heart disease mortality rates in Auckland, New Zealand, between 1982 and 1993. Circulation 2000;102:1511-6. [Crossref] [PubMed]
- Cooper R, Cutler J, Desvigne-Nickens P, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation 2000;102:3137-47. [Crossref] [PubMed]
- Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med 2007;356:2388-98. [Crossref] [PubMed]
- Tu JV, Nardi L, Fang J, et al. National trends in rates of death and hospital admissions related to acute myocardial infarction, heart failure and stroke, 1994-2004. CMAJ 2009;180:E118-25. [Crossref] [PubMed]
- Preis SR, Hwang SJ, Coady S, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 2009;119:1728-35. [Crossref] [PubMed]
- Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 2007;115:1544-50. [Crossref] [PubMed]
- Levi F, Lucchini F, Negri E, et al. Trends in mortality from cardiovascular and cerebrovascular diseases in Europe and other areas of the world. Heart 2002;88:119-24. [Crossref] [PubMed]
- Reddy KS. Cardiovascular disease in non-Western countries. N Engl J Med 2004;350:2438-40. [Crossref] [PubMed]
- Okrainec K, Banerjee DK, Eisenberg MJ. Coronary artery disease in the developing world. Am Heart J 2004;148:7-15. [Crossref] [PubMed]
- Montagnana M, Lippi G, Franchini M, et al. Sudden cardiac death: prevalence, pathogenesis, and prevention. Ann Med 2008;40:360-75. [Crossref] [PubMed]
- Kannel WB, Thomas HE Jr. Sudden coronary death: the Framingham Study. Ann N Y Acad Sci 1982;382:3-21. [Crossref] [PubMed]
- Zheng ZJ, Croft JB, Giles WH, et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation 2001;104:2158-63. [Crossref] [PubMed]
- Byrne R, Constant O, Smyth Y, et al. Multiple source surveillance incidence and aetiology of out-of-hospital sudden cardiac death in a rural population in the West of Ireland. Eur Heart J 2008;29:1418-23. [Crossref] [PubMed]
- Andréoletti L, Ventéo L, Douche-Aourik F, et al. Active Coxsackieviral B infection is associated with disruption of dystrophin in endomyocardial tissue of patients who died suddenly of acute myocardial infarction. J Am Coll Cardiol 2007;50:2207-14. [Crossref] [PubMed]
- Gillum RF. Sudden coronary death in the United States: 1980-1985. Circulation 1989;79:756-65. [Crossref] [PubMed]
- Kannel WB, Wilson PW, D'Agostino RB, et al. Sudden coronary death in women. Am Heart J 1998;136:205-12. [Crossref] [PubMed]
- Berger CJ, Murabito JM, Evans JC, et al. Prognosis after first myocardial infarction. Comparison of Q-wave and non-Q-wave myocardial infarction in the Framingham Heart Study. JAMA 1992;268:1545-51. [Crossref] [PubMed]
- Kannel WB, Cupples LA, D'Agostino RB. Sudden death risk in overt coronary heart disease: the Framingham Study. Am Heart J 1987;113:799-804. [Crossref] [PubMed]
- Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002;105:1897-903. [Crossref] [PubMed]
- Torp-Pedersen C, Kober L. Effect of ACE inhibitor trandolapril on life expectancy of patients with reduced left-ventricular function after acute myocardial infarction. TRACE Study Group. Trandolapril Cardiac Evaluation. Lancet 1999;354:9-12. [Crossref] [PubMed]
- Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death. Structure, function, and time-dependence of risk. Circulation 1992;85:I2-10. [PubMed]
- Chugh SS, Uy-Evanado A, Teodorescu C, et al. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The Ore-SUDS (Oregon Sudden Unexpected Death Study). J Am Coll Cardiol 2009;54:2006-11. [Crossref] [PubMed]
- Kim C, Fahrenbruch CE, Cobb LA, et al. Out-of-hospital cardiac arrest in men and women. Circulation 2001;104:2699-703. [Crossref] [PubMed]


