Proton pump inhibitors and risk of dementia
Introduction
Proton pump inhibitors (PPIs) are one of the most commonly prescribed medications in the United States (US) for treatment of several upper gastrointestinal disorders including gastroesophageal reflux and peptic ulcer (1). Over the past decade, the prevalence of the use of PPIs in the US has increased from 4.8% to 8.5% among women and from 3.4% to 7.0% among men (2). PPIs are generally regarded as safe medications with very few adverse effects. However, recent observational studies have suggested that some of its adverse effects, such as acute interstitial nephritis and Clostridium difficile infection, could be more common than previously thought (1,3-5).
Dementia is a disorder of older adults characterized by a decline in one or more cognitive functions (6). Known risk factors for dementia include diabetes mellitus, midlife hypertension, obesity, smoking, depression, physical inactivity, and cognitive inactivity (7). Use of PPIs could potentially be another risk factor for dementia and cognitive decline as demonstrated in recent observational studies even though the results were inconsistent (8-11). To summarize all available evidence and to further characterize this possible association, we conducted this systematic review and meta-analysis.
Methods
Search strategy
Two investigators (Karn Wijarnpreecha and Patompong Ungprasert) independently searched for published studies indexed in MEDLINE and EMBASE database from inception to April 2016 using the search strategy that included the terms for “PPIs” and “dementia” as described in online supplementary data (Supplementary 1). No language limitation was applied. A manual search for additional relevant studies using references from retrieved articles was also performed.
Inclusion criteria
The inclusion criteria were as follows: (I) case-control, cross-sectional or cohort studies published as original studies to evaluate the risk of dementia among subjects who used PPIs compared to non-users; (II) odds ratios (OR), risk ratios (RR), hazard ratios (HR) or standardized incidence ratio with 95% confidence intervals (CI) were provided.
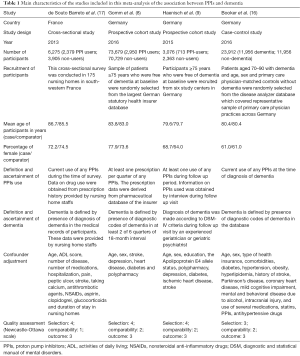
Study eligibility was independently determined by the two investigators noted above. Differences in the determination of study eligibility were resolved by mutual consensus. The quality of each study was also independently evaluated by each investigator using Newcastle-Ottawa quality assessment scale (12). This scale evaluated each study in three domains including the selection of the participants, the comparability between the groups and the ascertainment of the exposure for case-control study and the outcome of interest for cohort study. The modified Newcastle-Ottawa scale as described by Herzog et al. was used for cross-sectional study (13).
Data extraction
A standardized data collection form was used to extract the following data from each study: title of the study, name of the first author, year of study, year of publication, country of origin, number of participants, demographic data of participants, method used to identify and verify use of PPIs as well as dementia, adjusted effect estimates with 95% CI and covariates that were adjusted in the multivariate analysis.
To ensure the accuracy, this data extraction process was independently performed by all investigators. Any data discrepancy was also resolved by referring back to the original articles.
Statistical analysis
Data analysis was performed using Review Manager 5.3 software from the Cochrane Collaboration (London, UK). Adjusted point estimates and standard errors from individual study were combined by the generic inverse variance method of DerSimonian and Laird, which assigned the weight of each study based on its variance (14). In light of the high likelihood of between study variance because of different study designs and populations, we used a random-effect model rather than a fixed-effect model. Cochran’s Q test and I2 statistic were used to determine the between-study heterogeneity. A value of I2 of 0% to 25% represents insignificant heterogeneity, more than 25% but less than or equal to 50% represents low heterogeneity, more than 50% but less than or equal to 75% represents moderate heterogeneity, and more than 75% represents high heterogeneity (15).
Results
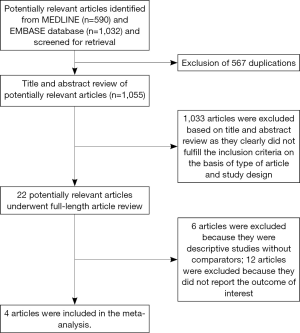
Our search strategy yielded 1,620 potentially relevant articles (590 articles from Medline and 1,032 articles from EMBASE). After the exclusion of 567 duplicated articles, 1,055 of them underwent title and abstract review. One thousand and thirty three articles were excluded at this stage since they were case reports, letters, review articles or interventional studies, leaving 22 articles for a full-length article review. Twelve of them were excluded since they did not report the outcome of interest while six articles were excluded since they were descriptive studies without comparators. Four articles (two cohort studies, one case-control study, and one cross-sectional study) met the eligibility criteria and were included in the data analysis (8,9,16,17). Figure 1 outlines the literature review and study selection process. The clinical characteristics and the quality assessment of the included studies are described in Table 1.
Full table
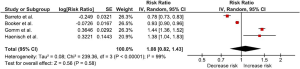
We found that PPIs users had a small increased risk of dementia compared with non-users with the pooled RR of 1.08 even though without a statistical significance (95% CI, 0.82–1.43). The statistical heterogeneity was high with an I2 of 99%. The forest plot is shown in Figure 2. However, sensitivity analysis that included only studies with high quality design (i.e., cohort studies) demonstrated a higher risk of dementia with the pooled RR of 1.44 (95% CI, 1.36–1.52). The statistical heterogeneity was minimal with an I2 of 0%.
Evaluation for publication bias
We did not perform the evaluation for publication bias as the number of studies included in the meta-analysis was too small.
Discussion
This study is the first systematic review and meta-analysis of published studies assessing the associations of the use of PPIs and risk of dementia. Overall, we found a small increased risk of dementia among PPIs users compared with non-users even though without reaching statistical significance. Nonetheless, sensitivity analysis of only cohort studies demonstrated a higher increased risk (approximately 40%) and achieved statistical significance.
There are few possible explanations for the apparent increased risk of dementia among PPIs users.
First, in vitro studies have demonstrated that PPIs could interfere with the degradation of amyloid beta (Aβ) peptide, one of the pathological hallmarks of Alzheimer’s disease (18). Fibrillar Aβ clearance by microglia is pH-dependent and induced by acidification of lysosomes. PPIs are known to have inhibitory effect on V-ATPase proton pump that is pivotal for acidification. Thus, use of PPIs might reduce the rate Aβ degradation, resulting in increased Aβ levels (19-21). Second, PPIs might act as an inverse γ-secretase modulator by increasing the activity of the β-secretase BACE1, resulting in accumulation of Aβ (22). Third, use of PPIs has been shown to be associated with vitamin B12 deficiency as a result of suboptimal GI absorption (23). Vitamin B12 deficiency is known to negatively affect cognitive function as a result of impaired DNA synthesis, methylation, and homocysteine neurotoxicity (24,25).
Although most of the included studies were of high quality as reflected by the high-quality assessment scores, this meta-analysis had some limitations. Therefore, the results should be interpreted with caution.
First, we could not perform the evaluation for publication bias as the number of included studies was too small. Thus, publication bias in favor of positive studies might have been present. Second, two studies included in this meta-analysis were medical registry-based studies which could raise a concern over coding inaccuracy and incompleteness. Third, the included studies were conducted exclusively in European countries. Therefore, our results might not be generalizable to other ethnic groups. Fourth, the statistical heterogeneity in this study was high. We suspect that the difference in study designs was responsible for this heterogeneity as the I2 dropped dramatically with the sensitivity analysis that included only cohort studies. Fifth, this is a meta-analysis of observational studies that could only demonstrate an association but could not establish causality. Therefore, we cannot conclude that PPIs use does increase the risk of dementia as this association could be a result of confounding.
Conclusions
In summary, this meta-analysis demonstrated an increased risk of dementia among PPIs users. Nonetheless, there are some limitations in methodology and the results should be interpreted with caution.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Search strategy
Database: Ovid MEDLINE
- exp proton pump inhibitor/
- proton pump inhibitor.mp.
- proton pump antagonist.mp.
- prton-translocating atpases.mp.
- proton pump.mp.
- ppi.mp.
- ppis.mp.
- lansoprazole.mp.
- dexlansoprazole.mp.
- kapidex.mp.
- prevacid.mp.
- omeprazole.mp.
- esomeprazole.mp.
- nexium.mp.
- prilosec.mp.
- pantoprazole.mp.
- protonix.mp.
- rabeprazole.mp.
- aciphex.mp.
- dexrabeprazole.mp.
- Pariet.mp.
- (I) or (II) or (III) or (IV) or (V) or (VI) or (VII) or (VIII) or (IX) or (X) or (XI) or (XII) or (XIII) or (XIV) or (XV) or (XVI) or (XVII) or (XVIII) or (XIX) or (XX) or (XXI)
- dementia.mp. or exp dementia/
- vascular dementia.mp or exp vascular dementia/
- exp multi-infarct/
- Alzheimer disease.mp. Or exp Alzheimer disease/
- cognitive decline.mp.
- cognitive impairment.mp.
- (XXIX) (XXIII) or (XXIV) or (XXV) or (XXVI) or (XXVII) or (XXVIII)
- (XXX) (XXII) and (XXIX)
References
- Schoenfeld AJ, Grady D. Adverse Effects Associated With Proton Pump Inhibitors. JAMA Intern Med 2016;176:172-4. [Crossref] [PubMed]
- Kantor ED, Rehm CD, Haas JS, et al. Trends in Prescription Drug Use Among Adults in the United States From 1999-2012. JAMA 2015;314:1818-31. [Crossref] [PubMed]
- Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open 2015;3:E166-71. [Crossref] [PubMed]
- Kwok CS, Arthur AK, Anibueze CI, et al. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol 2012;107:1011-9. [Crossref] [PubMed]
- Moledina DG, Perazella MA. Proton Pump Inhibitors and CKD. J Am Soc Nephrol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Kawas CH. Clinical practice. Early Alzheimer's disease. N Engl J Med 2003;349:1056-63. [Crossref] [PubMed]
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819-28. [Crossref] [PubMed]
- Gomm W, von Holt K, Thomé F, et al. Association of Proton Pump Inhibitors With Risk of Dementia: A Pharmacoepidemiological Claims Data Analysis. JAMA Neurol 2016;73:410-6. [Crossref] [PubMed]
- Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci 2015;265:419-28. [Crossref] [PubMed]
- Wise J. Proton pump inhibitors may be linked to dementia risk. BMJ 2016;352:i972. [Crossref] [PubMed]
- Corsonello A, Maggio M, Fusco S, et al. Proton pump inhibitors and functional decline in older adults discharged from acute care hospitals. J Am Geriatr Soc 2014;62:1110-5. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Herzog R, Álvarez-Pasquin MJ, Díaz C, et al. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013;13:154. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Booker A, Jacob LE, Rapp M, et al. Risk factors for dementia diagnosis in German primary care practices. Int Psychogeriatr 2016;28:1059-65. [Crossref] [PubMed]
- de Souto Barreto P, Lapeyre-Mestre M, Mathieu C, et al. Prevalence and associations of the use of proton-pump inhibitors in nursing homes: a cross-sectional study. J Am Med Dir Assoc 2013;14:265-9. [Crossref] [PubMed]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 2010;362:329-44. [Crossref] [PubMed]
- Fallahzadeh MK, Borhani Haghighi A, Namazi MR. Proton pump inhibitors: predisposers to Alzheimer disease? J Clin Pharm Ther 2010;35:125-6. [Crossref] [PubMed]
- Majumdar A, Cruz D, Asamoah N, et al. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell 2007;18:1490-6. [Crossref] [PubMed]
- Mattsson JP, Väänänen K, Wallmark B, et al. Omeprazole and bafilomycin, two proton pump inhibitors: differentiation of their effects on gastric, kidney and bone H(+)-translocating ATPases. Biochim Biophys Acta 1991;1065:261-8. [Crossref] [PubMed]
- Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One 2013;8:e58837. [Crossref] [PubMed]
- Lam JR, Schneider JL, Zhao W, et al. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013;310:2435-42. [Crossref] [PubMed]
- O'Leary F, Allman-Farinelli M, Samman S. Vitamin B12 status, cognitive decline and dementia: a systematic review of prospective cohort studies. Br J Nutr 2012;108:1948-61. [Crossref] [PubMed]
- Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol 2006;5:949-60. [Crossref] [PubMed]