Surgical pathology of early stage non-small cell lung carcinoma
Introduction
Non-small cell lung carcinomas (NSCLCs) have historically included adenocarcinoma (ADC), squamous cell carcinoma (SCC) and large cell carcinoma, the last being increasingly rare with improved ancillary techniques (1). Advances in chemotherapy and molecular targeted therapy, in particular, have made accurate subclassification essential for treatment. ADC is currently the most common histologic subtype of NSCLC and following the 1995 landmark study by Noguchi, et al. (2), much focus has been directed toward subtyping of ADCs in regard to prognostic significance, ultimately culminating in a comprehensive update of the histologic classification of ADC in 2011, which has subsequently been incorporated into the 2015 World Health Organization (WHO) classification of lung tumors (1). This classification scheme is based almost exclusively on studies of small ADCs less than three centimeters (2-6). As such, while the prognostic significance of the classification in larger tumors has not been well validated, its value has otherwise been supported in several studies focusing on smaller tumors (7). As with any classification system, additional issues potentially impacting prediction of tumor behavior and prognosis have arisen and need to be addressed. Additionally, the evolution of lung cancer screening programs with increased detection of small tumors, combined with an ongoing debate regarding the efficacy of sublobar resections for small tumors, increases the need for reliable prognostic markers for non-small cell carcinomas.
This paper will focus primarily on ADCs. The updated classification will be reviewed with focus on prognostic subgroups, followed by evolving issues regarding particular patterns of adenocarcinoma with poor prognostic implications, namely micropapillary and solid patterns as well as the recently described concept of “spread of tumor through alveolar spaces” (STAS). Discussion of ADC will be limited to focus on the most commonly encountered subtypes presenting as discrete tumors in patients with early stage disease. Tumors which are either very rare (i.e., fetal ADC) or more typically present as non-discrete pneumonic spread (invasive mucinous ADC, colloid ADC) will not be discussed. Literature focusing on prognostic features in SCC is much more limited at this time but will be addressed briefly. Finally, the implications of histology in regard to the issue of sublobar resections will be addressed.
Squamous cell carcinoma
Unlike ADC, SCC does not currently have a classification system that permits prognostic significance of predominant histologic subtypes. The absence, presence, and degree of keratinization have traditionally defined the grading. In the current updated classification scheme, histologic subtypes have been divided into “keratinizing” and “non-keratinizing” subtypes but they are not associated with prognostic or clinical significance. Kadota et al. However, attempted to look at single cell invasion and tumor budding to assess possible favorable/unfavorable prognostic indicators of death and recurrence in patients with SCC. The prognostic significance of histology, as well as the parameters mentioned above, requires additional study and validation before patients with SCC can be stratified into prognostic and therapy groups (1,8).
Adenocarcinoma
Over the past decades, it has been recognized that ADCs comprise a heterogeneous groups of tumors with different behaviors. Prognosis and survival are influenced by various factors including histologic subtype, size of tumors, type of surgical resection, and molecular profile (1). The clinical heterogeneity of lung ADC has made pathologic classification challenging. In 1995, Noguchi et al. (2) recognized that tumors with pure lepidic growth had a 100% 5-year survival and this definition was subsequently used in the 1999 WHO as the definition for what was then termed bronchioloalveolar carcinoma (BAC) and was retained in the 2004 WHO classification (2,9). At that time, however, the majority of ADCs fell into the so-called “mixed subtype” of ADC, a category which provided little if any prognostic information to the clinician (9-11).
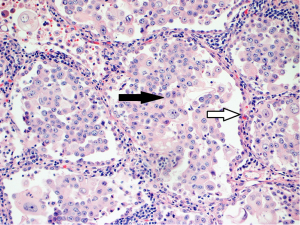
In attempt to address the need for a prognostically relevant classification, a multidisciplinary classification was published jointly by the American thoracic society (ATS), the European respiratory society (ERS) and the international association for the study of lung cancer (IASLC) in 2011, which was also adopted into the recently published 2015 WHO classification (1,9) (Table 1). In this classification, tumors with pure lepidic growth were reclassified as adenocarcinoma in situ (AIS) and the term BAC was eliminated. A new category of minimally invasive adenocarcinoma (MIA) was also introduced based upon data supporting that tumors with lepidic growth and an invasive component less than or equal to 5 mm have the same prognosis as those with pure lepidic growth. The mixed category was replaced with the recommendation that ADC be classified based on the predominant histologic subtype (lepidic, papillary, acinar, solid or micropapillary) (Figures 1-5), with a further recommendation that each component be recorded in 5% increments (1). The new categories of AIS and MIA have been proposed as Tis and T1a (mi) for the upcoming eight edition of the TNM classification of lung carcinoma (12).
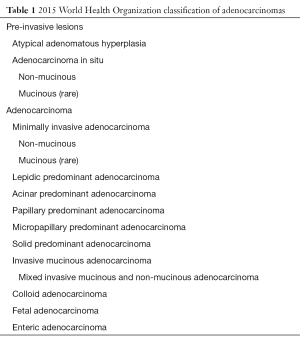
Full table
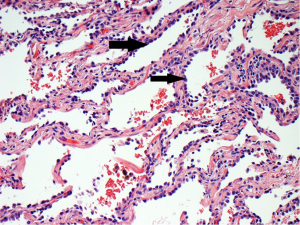
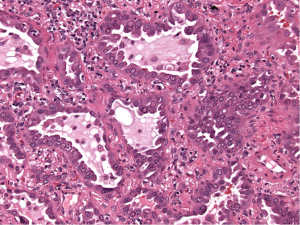
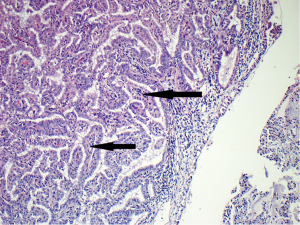
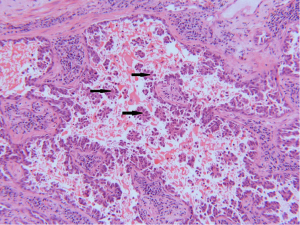
This new classification system stratifies tumors into morphological subgroups that have biologic relevance and may impact clinical decision-making. The prognostic significance of the histological classification system proposed by the IASLC has been validated by numerous studies and correlated with patient outcomes (3,7). While patients with AIS and MIA are reported to have 100% five-year disease free survival (DFS), the various histologic subtypes are associated with significant differences in DFS and overall survival (OS) (13-23). Overall, among the five invasive ADC subtypes comprised of lepidic, acinar, papillary, micropapillary, and solid predominant patterns, the lepidic predominant group had the best prognosis, papillary and acinar predominant intermediate prognosis, and solid and micropapillary predominant exhibited the poorest. Yoshizawa’s categorization by comprehensive histologic subtyping defined three overall prognostic groups by 5-year DFS. DFS for the low grade group, AIS and MIA, was 100%, intermediate grade comprised of non-mucinous lepidic predominant, papillary predominant, and acinar predominant achieved 90%, 83%, and 84% DFS, respectively, and finally, the high-grade histologic variants, including micropapillary predominant and solid predominant attained 67% and 70% DFS, respectively. Overall, there were significant differences in DFS among the low, intermediate, and high prognostic groups: 100%, 84% and 71%. Studies evaluating solid and micropapillary predominant tumors have demonstrated 5-year survival rates of 30–40% (13-18,20,24).
Molecular implications
Studies are ongoing to assess potential correlations between the updated classification system and the presence of certain targetable driver mutations, particularly EGFR and ALK. While there is a tendency for EFGR mutations to occur more frequently in lepidic, papillary, and micropapillary carcinomas, the latter particularly in Asian populations, EGFR mutations may be encountered in any of the histologic subtypes. Similarly, ALK rearrangements have been most commonly associated with the solid subtype, particularly if there is an associated signet ring component, but gene rearrangements have been reported in all subtypes (25-29).
Issues with current histologic classification system
While the updated ADC classification has shown correlation with outcome, as with any classification scheme, some issues require further clarification and investigation. As stated, the current recommendation is to classify ADC by the predominant subtype. However, this concept may not always be clear-cut. For example, in the “lepidic predominant” ADC, the lepidic component may be the predominant pattern present, but actually comprise less than 50% of the total tumor volume. In such cases, the predominant pattern present may be lepidic, but the majority of the tumor consists of various patterns of invasive carcinoma. At least one study has shown that tumors with greater than 50% lepidic growth and those with 10–50% have a 0% and 12% incidence of recurrence, respectively (30).
The percentage of micropapillary pattern needed to impact prognosis is also problematic in regard to behavior and prognosis. The inclusion of the micropapillary subtype was a significant addition to the updated classification. Histologically, this group consists of tumor cells that grow in papillary tufts lacking fibrovascular cores, in contrast to true papillary structures which contain fibrovascular cores. Numerous studies have highlighted the poor prognosis of this histologic pattern in lung carcinoma (13,14,22,31-36). As stated previously, studies have shown that solid and micropapillary predominant tumors have the worst prognosis among the histologic subtypes. Additional evidence suggests that the micropapillary pattern may in and of itself be a robust predictor of both prognosis and survival (14,20) and associated with poor outcomes (21,37). Furthermore, it has recently been suggested that the presence or absence of the micropapillary pattern may be an important prognostic indicator and impact survival (32-34). Rather than use the 5% increment as suggested by the IASLC classification, studies demonstrated that a micropapillary pattern of >1% of the tumor, resulted in metastasis and worse prognosis when compared to patients with no evidence of this histologic subtype (31). In addition, a micropapillary component of greater than 5% is associated with increased recurrence if treated by limited resection rather than lobectomy (22). Furthermore, this histologic subtype has been associated with a higher incidence of locoregional recurrence (38) and lymphovascular invasion (35,36).
A somewhat related issue is the recently described concept of tumor spread through air spaces (STAS). This pattern is defined as tumor cells spreading within air spaces in the lung parenchyma beyond the edge of the main tumor, in micropapillary structures without a central fibrovascular core, or in solid nests or tumor islands (Figure 6). While published literature is limited and further investigation is needed to clarify several issues, this pattern has been associated with poor outcomes and lymphatic invasion. The authors also raise the possibility that in patients with small percentages of micropapillary tumor (5%) who do poorly may progress because of “micropapillary STAS”. Of potential treatment significance, patients with STAS had a poorer prognosis only when they underwent limited resection, but not in those who underwent lobectomy, suggesting a role for lobectomy even in small lesions (39).
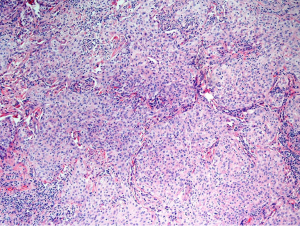
Solid predominant histological component has been associated with high grade clinical behavior. A 70% 5-year DFS has been associated with this aggressive subtype (13,40). Furthermore, it has been shown to be a predictor of early recurrence and poor post-recurrence survival. Patients with this histological subtype had the highest risk of recurrence and earlier recurrence than for intermediate and low-risk histologies with greater extra-thoracic and multisite spread. When compared to low and intermediate histological subtype groups, OS was significantly worse in solid predominant tumors. Furthermore, as these tumors tend to be EGFR negative and KRAS positive, they are not amenable to molecular targeting. For this cohort of patients, adjuvant therapeutic strategies require investigation (41).
Treatment implications of the new classification system
The histologic issues above are some of many which need to be addressed in the evolving evaluation of the efficacy of sublobar resection which once again has come to the forefront with the advent of CT testing and the increasing detection of small lesions radiographically. The standard of care for early stage lung ADC is lobectomy, but segmentectomies and wedge resections are now more commonly alternatives, particularly for small (<2 cm) lesions (42). Radiologic evidence has shown the wedge/segmentectomy may be appropriate for small tumors with a ground glass or predominantly ground glass appearance (43,44). These tumors for the most part correspond to AIS and MIA (38). While pure ground glass tumors have a 100% DFS survival after limited resection there are instances, albeit rare, where recurrences have occurred at the resection margin (44).
Studies to evaluate whether limited resection is appropriate for small tumors of mixed ground glass and solid subtypes are ongoing, as consistent conclusive evidence supporting limited resection for invasive tumors is still lacking. The issue of histologic subtype needs to be taken into consideration for these tumors in particular. Given the data demonstrating poor outcomes with aggressive histologic subtypes in small lesions, particularly with micropapillary histology, and studies demonstrating recurrence in patients with small lesions, sublobar resections (22) may need more careful consideration, particularly with small solid or subsolid radiographic lesions for which a micropapillary component may not be excluded. Further evaluation of the impact of micropapillary growth and STAS in sublobar resections is needed. Additionally, better information in regard to confounding variables such as margin distance (which can be difficult to evaluate grossly in both of these patterns) is needed.
Given that the presence of certain ADC subtypes may potentially impact the choice of surgical resection, the accuracy of identifying histologic patterns of ADC at the time of frozen section has received recent attention. Trejo Bittar et al., recently evaluated 112 consecutive surgically resected stage 1 lung ADCs in order to determine diagnostic accuracy and interobserver variability in histologic subtyping of lung ADCs at the time of frozen section (45). In this study, primary and secondary histologic patterns were assigned in each case by three pathologists independently, with comparison between interpretation of frozen section and permanent section results. In this study, kappa values ranged from 0.48–0.58 for the primary histologic pattern. Particular attention was also paid to the identification of a micropapillary pattern, which was recognized with a 98% specificity rate when identified but was recognized with only a 13% sensitivity rate. Similarly, Yeh et al., evaluated 361 resected stage 1 ADCs in regard to accuracy of pattern identification, and additionally attempted to evaluate the accuracy of the presence of invasion at the time of frozen section in 35 cases (46). Similar to the Trejo BIttar study, agreement on the predominant histologic subtype achieved only a moderate a kappa value of 0.565 and again showed high specificity but low sensitivity for the identification of a micropapillary component. In regard to evaluation of degree of invasion, the kappa value was only 0.378. Sampling issues and quality of frozen section slides were cited as the most common reasons for discrepancy. These results suggest that identification of ADC subtype by frozen section is not sufficiently reliable to serve as the sole basis for choice of surgical resection.
In regard to other treatment modalities, although adjuvant therapy is not typically used in the case of resectable low stage disease, given the varied diagnostic and prognostic outcomes for patients with different histologic subtypes, the new IASLC/ATS/ERS classification may provide a rationale for stratifying adjuvant chemotherapeutic treatment options among these different subgroups of patients. Brambilla et al. have shown that patients with micropapillary and solid histologic subtypes, both associated with poor DFS, obtained significant benefit from treatment with adjuvant chemotherapy when compared to less aggressive histologic subtypes (47).
Conclusions
In summary, the updated 2015 WHO classification of ADCs is a much improved classification in comparison to the 2004 WHO classification in regard to provided information regarding tumor prognosis. Several issues require further study, particularly micropapillary carcinoma and the related concept of STAS, in regard both to prognosis and the potential impact on choice of resection. SCC has been studied much less extensively than ADC and further evaluation of this tumor subtype is needed in regard to both prognosis and treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Borczuk AC, Qian F, Kazeros A. Invasive size is an independent predictor of survival in pulmonary adenocarcinoma. Am J Surg Pathol 2009;33:462-9. [Crossref] [PubMed]
- Yim J, Zhu LC, Chiriboga L, et al. Histologic features are important prognostic indicators in early stages lung adenocarcinomas. Mod Pathol 2007;20:233-41. [Crossref] [PubMed]
- Maeshima AM, Tochigi N, Yoshida A, et al. Histological scoring for small lung adenocarcinomas 2 cm or less in diameter: a reliable prognostic indicator. J Thorac Oncol 2010;5:333-9. [Crossref] [PubMed]
- Murakami S, Ito H, Tsubokawa N, et al. Prognostic value of the new IASLC/ATS/ERS classification of clinical stage IA lung adenocarcinoma. Lung Cancer 2015;90:199-204. [Crossref] [PubMed]
- Kadota K, Nitadori J, Woo KM, et al. Comprehensive pathological analyses in lung squamous cell carcinoma: single cell invasion, nuclear diameter, and tumor budding are independent prognostic factors for worse outcomes. J Thorac Oncol 2014;9:1126-39. [Crossref] [PubMed]
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. editors. Pathology & Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC, 2004.
- Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 2005;40:90-7. [Crossref] [PubMed]
- Kerr KM. Pulmonary adenocarcinomas: classification and reporting. Histopathology 2009;54:12-27. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM Stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [Crossref] [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Song Z, Zhu H, Guo Z, et al. Prognostic value of the IASLC/ATS/ERS classification in stage I lung adenocarcinoma patients—based on a hospital study in China. Eur J Surg Oncol 2013;39:1262-8. [Crossref] [PubMed]
- Gu J, Lu C, Guo J, et al. Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients-A single institution retrospective study of 292 lung adenocarcinoma. J Surg Oncol 2013;107:474-80. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol 2013;8:612-8. [Crossref] [PubMed]
- Woo T, Okudela K, Mitsui H, et al. Prognostic value of the IASLC/ATS/ERS classification of lung adenocarcinoma in stage I disease of Japanese cases. Pathol Int 2012;62:785-91. [Crossref] [PubMed]
- Zhang J, Wu J, Tan Q, et al. Why do pathological stage IA lung adenocarcinomas vary from prognosis?: A clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinoma based on the IASLC/ATS/ERS classification. J Thorac Oncol 2013;8:1196-202. [Crossref] [PubMed]
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [Crossref] [PubMed]
- Hung JJ, Jeng WJ, Chou TY, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg 2013;258:1079-86. [Crossref] [PubMed]
- Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105:1212-20. [Crossref] [PubMed]
- Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013;81:371-6. [Crossref] [PubMed]
- Song Z, Zhu H, Guo Z, et al. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol 2013;30:645. [Crossref] [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [Crossref] [PubMed]
- Mansuet-Lupo A, Bobbio A, Blons H, et al. The new histologic classification of lung primary adenocarcinoma subtypes is a reliable prognostic marker and identifies tumors with different mutation status: the experience of a French cohort. Chest 2014;146:633-43. [Crossref] [PubMed]
- Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012;18:1947-53. [Crossref] [PubMed]
- Lan TT, Brown NA, Hristov AC. Controversies and considerations in the diagnosis of primary cutaneous CD4+ small/medium T-cell lymphoma. Arch Pathol Lab Med 2014;138:1307-18. [Crossref] [PubMed]
- Rekhtman N, Ang DC, Riely GJ, et al. KRAS mutations are associated with solid growth pattern and tumor-infiltrating leukocytes in lung adenocarcinoma. Mod Pathol 2013;26:1307-19. [Crossref] [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [Crossref] [PubMed]
- Lee G, Lee HY, Jeong JY, et al. Clinical impact of minimal micropapillary pattern in invasive lung adenocarcinoma: prognostic significance and survival outcomes. Am J Surg Pathol 2015;39:660-6. [Crossref] [PubMed]
- Watanabe M, Yokose T, Tetsukan W, et al. Micropapillary components in a lung adenocarcinoma predict stump recurrence 8 years after resection: a case report. Lung Cancer 2013;80:230-3. [Crossref] [PubMed]
- Maeda R, Isowa N, Onuma H, et al. Lung adenocarcinomas with micropapillary components. Gen Thorac Cardiovasc Surg 2009;57:534-9. [Crossref] [PubMed]
- Cha MJ, Lee HY, Lee KS, et al. Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg 2014;147:921-928.e2. [Crossref] [PubMed]
- Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol 2003;27:101-9. [Crossref] [PubMed]
- Kamiya K, Hayashi Y, Douguchi J, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol 2008;21:992-1001. [Crossref] [PubMed]
- Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol 2010;34:1155-62. [Crossref] [PubMed]
- Nitadori J, Bograd AJ, Morales EA, et al. Preoperative consolidation-to-tumor ratio and SUVmax stratify the risk of recurrence in patients undergoing limited resection for lung adenocarcinoma ≤2 cm. Ann Surg Oncol 2013;20:4282-8. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 2008;32:810-27. [Crossref] [PubMed]
- Ujiie H, Kadota K, Chaft JE, et al. Solid predominant histologic subtype in resected stage I lung adenocarcinoma is an independent predictor of early, extrathoracic, multisite recurrence and of poor postrecurrence survival. J Clin Oncol 2015;33:2877-84. [Crossref] [PubMed]
- Dembitzer FR, Flores RM, Parides MK, et al. Impact of histologic subtyping on outcome in lobar vs sublobar resections for lung cancer: a pilot study. Chest 2014;146:175-81. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. [Crossref] [PubMed]
- Trejo Bittar HE, Incharoen P, Althouse AD, et al. Accuracy of the IASLC/ATS/ERS histological subtyping of stage I lung adenocarcinoma on intraoperative frozen sections. Mod Pathol 2015;28:1058-63. [Crossref] [PubMed]
- Yeh YC, Nitadori J, Kadota K, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of ≤ 3 cm: accuracy and interobserver agreement. Histopathology 2015;66:922-38. [Crossref] [PubMed]
- Brambilla EM, Marguet S, Le Teuff G, et al. Prognostic and predictive value of a new IASLC/ATS/ERS lung adenocarcinoma classification in a pooled analysis of four adjuvant chemotherapy trials: a LACE-BIO study. J Thorac Oncol 2013;8 Suppl 2:S201.


