The significance of tumor-associated immune response in molecular taxonomy, prognosis and therapy of colorectal cancer patients
Introduction
Colorectal cancer (CRC) is the third most common cancer in men (10% of all cancers) and the second in women (9.2% of all cancers) worldwide (1). Mortality rates remain high despite the fact that new operative and oncological therapies have been applied. Almost half of the patients undergoing treatment with curative intent will survive up to 5 years. Nevertheless, a substantial 25% of the conventionally classified [AJCC/UICC Tumor-Node-Metastasis (TNM) classification] stage I/II CRCs recur despite pathologically confirmed complete surgical resection and no evidence of residual tumor burden or distant metastases (2). The reason for that remains unclear but it definitely implies that the TNM classification does not have the needed accuracy for predicting outcome in all stages. Several other ways have been proposed to classify CRC. These rely on tumor cell characteristics, including morphology, molecular pathways, mutation status, cell origin and gene expression-based methods and allows the distinction of multiple, yet overlapping subtypes (3-7). This overlapping could be bypassed with classifications not based on the neoplastic cells and their characteristics but on the immunologic profile of the tumor microenvironment, also named tumor-associated immune response or host immune response (8). This is an important determinant of outcome in human cancers (9,10). The prognostic impact of immune cell varies based on the type of cancer but in general high densities of T cells (CD3+), cytotoxic T cells (CD8+) and memory T cells (CD45R0+) are clearly associated with longer disease-free survival (DFS) and overall survival (OS) (10). More than one hundred published studies confirm the prognostic value of the immune cell infiltrates in patients with CRC. The observed association with improved survival is regardless of pathological stage (11). Moreover, efforts have been made to clarify whether lymphocyte subtyping adds additional prognostic information beyond the evaluation of inflammatory cells on routine haematoxylin and eosin (H&E) stained sections (12).
In this review, we try to highlight the impact of tumor-associated immune responses in the prognosis of CRC patients and to present tools that can help distinguish those patient groups who would potentially benefit from future applied immunotherapies.
Methods for assessing immune response in colorectal cancer
To be used globally in a routine manner, evaluation of a novel marker should be simple, applicable in daily practice, feasible and inexpensive, reproducible, quantitative, standardized, pathology-based and powerful (13).
In order to improve the risk stratification for patients with CRC, many researchers have applied simple, specific T cell subtype immunohistochemistry-based density analysis. Density is graded as absent, weak, moderate or strong in three separate compartments: (I) invasive margin (IM); (II) tumor stroma (ST) and (III) cancer cell nests (CCN) (14).
Another immunohistochemistry-based score, the Galon’s “Immunoscore”, grades CD45R0 or CD3 and CD8 infiltration at both the IM and central tumor (CT) as either high (Hi) or low (Lo), according to the median number of positive cells. Patients are assigned to one of four prognostic groups depending on the total number of areas graded Hi or Lo (15).
A third method for assessing the local inflammatory response in CRC is the Klintrup-Makinen grade, an assessment of inflammatory cell infiltration only at the IM, using H&E stained sections. Patients are assigned to one of four prognostic groups depending on the intensity of the inflammatory cell reaction. Zero (0) denotes no increase of inflammatory cells, 1 denotes mild and patchy increase of inflammatory cells, 2 denotes a band-like infiltrate at the IM with some evidence of destruction of cancer cell islets and 3 denotes a very prominent inflammatory reaction with frequent destruction of cancer cells (16).
All methods seem to exhibit similar survival relationships in both node-positive and node-negative CRC, with a favorable prognostic impact of lymphocytic infiltrates (14).
“Immunoscore” versus TNM staging
The classification based only on tumor invasion parameters, has been shown to be valuable in estimating the outcome of patients in a variety of cancers (17). However, clinical outcome can significantly vary among patients within the same histological type and tumor stage (2,18). Histopathological analysis of colorectal tumors has revealed varying infiltration by inflammatory and lymphocytic cells (19). In depth intra-tumor analysis reveals that these immune infiltrates are not randomly distributed. Tumor-infiltrating immune cells appear to be localized and organized within dense infiltrates in the CT and at the IM of tumor nests. An underlying biology may be reflected by this immune reaction, as revealed by gene expression profiling and other assays. These gene-signature sets include evidence for innate immune activation, secretion of chemokines for innate and adaptive cell recruitment, expression of immune effector molecules and immunoregulatory factors (20,21). Large cohort studies (with sample sizes of 843 and 768 patients, respectively) have revealed that tumor immune infiltrate patterns in CRC are significant prognostic biomarkers, even after adjusting for stage, lymph node count and well-established prognostic tumor molecular biomarkers, including microsatellite instability (MSI) and BRAF mutations (22,23). When the “Immunoscore” was tested in two large independent cohorts (n=602), only 4.8% of patients, among those harbouring tumors with a high “I”4, relapsed after 5 years. In contrast, 72% of patients with a low tumor Immunoscore (“I”0 and “I”1) had relapsed and only 27.5% were alive at 5 years. Similar results have been obtained from other studies, illustrating that these “I”0 and “I”1 patients could have potentially benefited from adjuvant therapy (2,15,20,24,25). Mlecnik et al. showed that in CRC patients with TNM stages I/II/III, the best predictor for outcome among all clinical parameters was the Immune score. In Cox multivariate regression analysis, after adding the AJCC/UICC-TNM stages and the Immune score in the model, only the Immune score remained significantly associated with disease-specific survival and OS (HR 0.63 and 0.71, respectively, all P<0.001). Results were also confirmed in an independent cohort of 184 patients (Immune score: HR 0.42 and 0.64, respectively, all P<0.001) (15).
Immune response gene expression in colorectal cancer
Pentheroudakis et al. studied mRNA expression of seven immune response-related genes (CD3Z, CD8, CD4, CXCL9, CXCL13, IGHM and FOXP3) in patients with stage II and III colorectal tumors managed with oxaliplatin-based adjuvant chemotherapy and came to the conclusion that (10,26,27) only CD3 and CD8 can cluster the CRC patients into distinctive “mRNA-based Immune Score” high versus low (mIS) cases. CD3 is a marker of activated T lymphocytes, encoding a protein which forms the T cell receptor-CD3 complex, important for coupling antigen recognition to several intracellular signal-transduction pathways. CD8 encodes a cell surface glycoprotein found on most cytotoxic T lymphocytes that mediates efficient cell-cell interaction with class I MHC antigen-presenting cells. However, the prognostic significance of mIS was restricted to a specific tumor stage and site. Specifically, only in patients with stage III right-sided colon cancer, a low immune response was associated with significantly inferior DFS (mIS-low, HR 2.28, 95% CI: 1.05-8.02) (28), while no prognostic impact was seen in left-sided tumors. In addition, a recently published novel 12-gene immune signature that was generated from miRNA/mRNA expression analysis, was shown to be an independent factor in predicting OS, as well as DFS in CRC patients (29).
In a more integrated analysis, spatio-temporal dynamics of intra-tumoral immune cells revealed that patients with diverse gene expression patterns have different clinical outcomes. Genes highly expressed in patients with prolonged DFS were related to cytotoxic T cell surface molecules, T helper cell surface molecules and chemokine-related functions associated to endothelial cell migration. In contrast, for patients with unfavorable outcome, an overexpression of genes with a role in IL-2 signaling and in the downregulation of adaptive immune responses was observed. This process seems to be complex and according to Bindea et al. evolves at each tumor stage. Densities of T follicular helper cells and innate cells increase, whereas most effector T cell densities decrease with tumor progression. The numbers of B cells increase at a late stage and have a dual effect, tumor-promoting and tumor-suppressive, depending on the complex fine regulation of the immune contexture. Chemokines, such as CXCL13 and IL-21 have a pivotal role in shaping the effective anti-tumor immune reaction in CRC (30).
Immune response and correlation with clinicopathological factors
In the Mlecnik et al. study the pT (depth of tumor) and pN stage (lymph node invasion), as well as the presence of bowel perforation were the clinical parameters significantly associated with survival. However, the Immune score was found to be the best predictor among all clinical parameters. In multivariate analysis only the Immune score remained significant for prognosis (15).
Various hypotheses have been generated for the impact of molecular processes on the intensity and nature of the host immune response. CRC can molecularly be divided into three groups: (I) chromosomal unstable (CIN); (II) microsatellite unstable (MSI); and (III) CpG island methylator phenotype (CIMP). Most of the cases arise through the CIN pathway, with various degrees of chromosomal number alterations and loss of heterozygosity. Mutations in specific tumor suppressor genes and oncogenes that activate pathways critical for CRC initiation and progression are accumulated in these cancers. Hypermutation characterizes the microsatellite unstable (MSI-high) CRCs, which represent 15% of all CRCs. Frame-shift mutations in MSI-high CRCs constitute a potential source of targetable neo-antigens (31). Epigenetic silencing of a mismatch-repair gene (MLH1) causes most of the MSI-high cancers [mismatch repair deficient (dMMR)] (6,32,33). This phenomenon occurs mostly in tumors of the CpG island methylator phenotype (CIMP-positive), though not all CIMP cases result in MLH1 promoter methylation. Consequently, a classification overlap occurs (6,33), while the degree of the host immune reaction varies in these overlapping subtypes.
dMMR tumors often contain intra-epithelial T cells in response to the expression of neo-antigens on the cell surface (34). This could be the explanation for the better prognosis observed in patients with dMMR tumors. Additionally, analysis of a cohort of 1197 patients confirmed the prognostic value of CD3+, CD8+ and CD45R0+ T cell infiltration in proficient-MMR (pMMR) CRCs (35). Other studies showed improved survival in patients with dMMR tumors and high density of cytotoxic CD3+ lymphocytes (36,37). In another large study with 768 CRCs, dMMR tumors were positively associated with CD45R0+ cell density, although the survival benefit associated with tumor infiltrating CD45R0+ cells was independent of MSI status (22). Accumulating data suggest that complex factors regulate the connection of the MSI status with that of the host immune response.
Discordant classification problems could potentially be solved by a consensus gene expression-based classification system for CRC, taking into account all the key components linked to tumor, stroma and host cellular functions. A group of experts recently published in Nature Medicine a proposal, after showing interconnectivity between six independent classification systems. Significant biological differences in the consensus molecular subtypes (CMS) support a new taxonomy for this disease with distinct molecular groups: (I) CMS1 (MSI immune) with a hypermutated profile, microsatellite unstable, CIMP-positive with strong immune activation; (II) CMS2 (canonical) with epithelial, marked WNT and MYC signalling activation and frequent somatic copy number aberrations; (III) CMS3 (metabolic) with epithelial, evident metabolic dysregulation and frequent KRAS mutations; and (IV) CMS4 (mesenchymal) with prominent transforming growth factor-β activation, stromal invasion and angiogenesis. About 10–15% of CRCs represent a transitional phenotype and cannot be classified. The authors identified no specific gene aberrations reliably identifying any CMS type, apart from the nearly universal genetic activation of the receptor tyrosine kinase and mitogen-activated protein kinase pathways in CMS1 and CMS3. This supports the notion that tumors harbouring commonly assumed driver events in CRC still vary markedly in their biology, highlighting the very poor genotype/phenotype correlations in CRC. Important associations between the CMS groups and clinical variables have been observed. CMS1 tumors are frequently diagnosed in females with right-sided lesions and present with higher histopathological grade. On the other hand, CMS2 tumors are mainly left sided. Finally, CMS4 tumors have a tendency to be more advanced (stages III and IV). In multivariate analyses, after adjustment for clinicopathological features, MSI status and presence of BRAF or KRAS mutations, CMS4 tumors had a worse OS and relapse-free survival. Superior survival rates after relapse have been found in the group of CMS2 patients (38). Of interest, the CMS1 population had a very poor survival rate after relapse, in agreement with recent studies showing worse prognosis for patients with dMMR and BRAF-mutated CRCs that recur (39-41).
The group of patients harboring MSI-high and BRAF mutated tumors with strong immunity could potentially benefit from immunological therapeutic interventions. On the other hand, CRC patients with RAS mutant tumors had significantly lower expression of a coordinated immune response, a fact that could be translated into unsuccessful immunological therapies (42).
Immune response and therapeutic implications
Chemotherapeutics like oxaliplatin, commonly used in 5-FU-based regimens, can stimulate a highly potent immune response by increasing neo-antigen release and presentation via antigen presenting cells (APCs), with enhancement of T cell responses and generation of memory T cells (43,44). Tumor cells can be susceptible to cytotoxic T lymphocytes by upregulation of “death receptors”, such as FAS or TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) (45). Correale et al. reported a better outcome in advanced CRC patients treated with oxaliplatin chemotherapy or chemo-immunotherapy if previously an intense T-regulatory cell (Treg) infiltration was present in primary tumors, suggesting a reversal of immune suppression (46). These immunogenic effects of oxaliplatin on the host immune response could transform its conventional use as an empiric cytotoxic drug to an immunomodulatory drug that can be used in combination with potent immunotherapies in order to potentiate their effect.
Despite the fact that immunotherapies have been proven successful in other types of cancer, the majority is being evaluated in early-phase (phase I and II) clinical trials for CRC. Current immunotherapies for CRC fall into 8 broad categories: (I) monoclonal antibodies (MAbs); (II) checkpoint inhibitors and immune modulators (anti-CTLA-4, anti-PD-1, anti-PD-L1); (III) cancer vaccines; (IV) adoptive cell therapy; (V) dendritic cell therapies; (VI) oncolytic virus therapy; (VII) cytokines; (VIII) adjuvant immunotherapies (47). For the purposes of this review we only report immunotherapies with clinically significant results in phase II, as well as ongoing phase II and III CRC trials. All ongoing trials are summarized in Table 1.
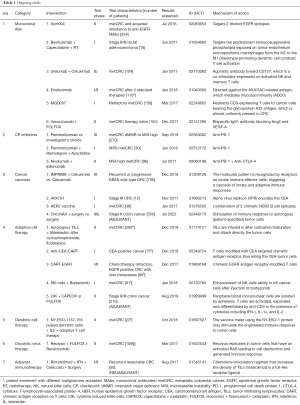
Full table
Agents that target immune-checkpoint pathways, such as PD-1, PD-L1 and CTLA-4 have shown to have objective and durable responses in different types of tumors like melanoma, non-small cell lung cancer and renal-cell carcinomas. PD-1 is expressed on a large proportion of tumor infiltrating lymphocytes and when bound to its ligands, PD-L1 and PD-L2, leads to lymphocyte anergy. Chronic antigen exposure and various escape mechanisms hijacked by cancer can lead to high levels of PD-L1 expression on host, stromal and tumor cells, as assessed by immunohistochemistry. Compared to peripheral blood, PD-1 is upregulated in CD8+ T cells. In patients with localized CRC, PD-L1 expression was observed in 37% of pMMR and in 29% of dMMR CRCs (48). In other groups, PD-L1 expression was observed in 38% of dMMR but only in 13% of pMMR CRCs (49). In a recently presented phase II study, evaluating pembrolizumab (an anti-PD-1 monoclonal antibody), previously treated patients with dMMR CRC tumors had an overall response rate (ORR) of 62% and a disease control rate (DCR) of 92%. In sharp contrast, pMMR CRC patients had inferior responses (0% ORR and 16% DCR). At one-year median follow-up, the dMMR group maintained high response rates with median PFS and OS not reached, in contrast to the pMMR group (median PFS 2.3 months, median OS 5 months). Interestingly, dMMR tumors were highly mutated with approximately 1700 mutations vs. 70 mutations per tumor in pMMR cases (P=0.007). The mutational burden was significantly associated with efficacy (P=0.02) (50). The authors have recently announced the initiation of a phase III trial (named KEYNOTE-177) with pembrolizumab versus investigator-choice chemotherapy in MSI-high or dMMR stage IV CRC (NCT02563002). Another phase II clinical trial of nivolumab (anti PD-1) versus nivolumab combined with ipilimumab (anti-CTLA-4) in recurrent and metastatic MSI-high CRC is ongoing with first results expected at the beginning of 2017 (NCT02060188).
Antigen presenting cells (APCs) may also trigger the adaptive immune response by increasing the priming and the cytotoxic effect of tumor-specific CD8+ lymphocytes. Toll-like receptor agonists are able to activate APCs in this way. Conjugation to cetuximab (a MAb targeting the EGFR in RAS wild-type CRC) increases the innate signalling and the antitumoral effect of cetuximab (51). Similar boost-like immune effects by the so called “adjuvant immunotherapies” are under investigation with phase II clinical trials enrolling recurrent, irresectable CRC patients with a chemokine modulatory regimen combining IFN, celecoxib and rintatolimod (NCT01545141).
Imprime PCG is a vaccine that conjugates the innate with the adaptive immune response. This vaccine works synergistically with anti-tumor MAbs like cetuximab. Results from a phase II clinical trial showed doubling of overall responses that led to a phase III trial (PRIMUS), where first results are expected during 2016 (NCT01309126). While some immune-modulatory drugs trigger a broad pro-inflammatory response, imprime PCG selectively targets and activates neutrophils without inducing systemic pro-inflammatory cytokines that are attributed to adverse reactions (52).
Oncolytic virus therapy uses a modified virus that can cause tumor cells to self-destruct and generate a greater immune response against the cancer. Reolysin is such a virus therapy, which is especially effective in RAS-activated tumors (53). A randomized phase II study of reolysin in combination with FOLFOX plus bevacizumab versus FOLFOX plus bevacizumab alone in patients with metastatic CRC is underway and primary results are expected at the beginning of 2017 (NCT01622543).
Conflicting results have been presented on adjuvant therapy with the monoclonal antibody edrecolomab, which can potentially restore the immune responses of patients with resected CRC. One study showed improved survival (54), while two more randomized trials failed to reproduce any survival benefit (55,56).
Conclusions
Overall results of studies on immunotherapies for CRC patients yielded conflicting, or only preliminary data. In order to clarify the real effect of immune therapies, predictive biomarkers, able to identify CRC patients who might benefit from those patients with resistant tumors, need to be identified and validated. Moreover, insights in the function and regulation of the tumor host immune interaction need to be generated and studied. Encouraging, ongoing network efforts are heading to that direction.
It remains unclear what the immunological profile of the metastatic disease might be and if that might have a correlation to the primary tumor profile (57). The immunological therapeutic implications might have a critical impact only in the adjuvant setting and mainly in those patients with early stage disease, as immune escape mechanisms prevail in the metastatic tumor making it difficult for the immune response to control the malignancy. Nevertheless, recent data suggest that immune-modulating therapies hold promise for patients with advanced disease, with minimal normal tissue toxicity, highlighting the dynamic and powerful potential of the host immune system. Future research efforts will likely focus on devising more elaborate ways to manipulate the host immune response and on combining immunomodulation with chemotherapy and targeted therapies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Mlecnik B, Bindea G, Pagès F, et al. Tumor immunosurveillance in human cancers. Cancer Metastasis Rev 2011;30:5-12. [Crossref] [PubMed]
- De Sousa E, Melo F, Wang X, Jansen M, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med 2013;19:614-8. [Crossref] [PubMed]
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-65. [Crossref] [PubMed]
- Nosho K, Irahara N, Shima K, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One 2008;3:e3698. [Crossref] [PubMed]
- Ogino S, Cantor M, Kawasaki T, et al. CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut 2006;55:1000-6. [Crossref] [PubMed]
- Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut 2009;58:90-6. [Crossref] [PubMed]
- Ogino S, Galon J, Fuchs CS, et al. Cancer immunology--analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol 2011;8:711-9. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298-306. [Crossref] [PubMed]
- Roxburgh CS, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev 2012;38:451-66. [Crossref] [PubMed]
- Huh JW, Lee JH, Kim HR. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch Surg 2012;147:366-72. [Crossref] [PubMed]
- Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol 2014;232:199-209. [Crossref] [PubMed]
- Richards CH, Roxburgh CS, Powell AG, et al. The clinical utility of the local inflammatory response in colorectal cancer. Eur J Cancer 2014;50:309-19. [Crossref] [PubMed]
- Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011;29:610-8. [Crossref] [PubMed]
- Klintrup K, Mäkinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer 2005;41:2645-54. [Crossref] [PubMed]
- Weitz J, Koch M, Debus J, et al. Colorectal cancer. Lancet 2005;365:153-65. [Crossref] [PubMed]
- Nagtegaal ID, Quirke P, Schmoll HJ. Has the new TNM classification for colorectal cancer improved care? Nat Rev Clin Oncol 2011;9:119-23. [Crossref] [PubMed]
- Finn OJ. Cancer immunology. N Engl J Med 2008;358:2704-15. [Crossref] [PubMed]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [Crossref] [PubMed]
- Wang E, Miller LD, Ohnmacht GA, et al. Prospective molecular profiling of melanoma metastases suggests classifiers of immune responsiveness. Cancer Res 2002;62:3581-6. [PubMed]
- Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol 2010;222:350-66. [Crossref] [PubMed]
- Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res 2009;15:6412-20. [Crossref] [PubMed]
- Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009;27:5944-51. [Crossref] [PubMed]
- Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005;353:2654-66. [Crossref] [PubMed]
- Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer 2014;110:1595-605. [Crossref] [PubMed]
- Sherwood AM, Emerson RO, Scherer D, et al. Tumor-infiltrating lymphocytes in colorectal tumors display a diversity of T cell receptor sequences that differ from the T cells in adjacent mucosal tissue. Cancer Immunol Immunother 2013;62:1453-61. [Crossref] [PubMed]
- Pentheroudakis G, Raptou G, Kotoula V, et al. Immune response gene expression in colorectal cancer carries distinct prognostic implications according to tissue, stage and site: a prospective retrospective translational study in the context of a hellenic cooperative oncology group randomised trial. PLoS One 2015;10:e0124612. [Crossref] [PubMed]
- An N, Shi X, Zhang Y, et al. Discovery of a Novel Immune Gene Signature with Profound Prognostic Value in Colorectal Cancer: A Model of Cooperativity Disorientation Created in the Process from Development to Cancer. PLoS One 2015;10:e0137171. [Crossref] [PubMed]
- Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013;39:782-95. [Crossref] [PubMed]
- Williams DS, Bird MJ, Jorissen RN, et al. Nonsense mediated decay resistant mutations are a source of expressed mutant proteins in colon cancer cell lines with microsatellite instability. PLoS One 2010;5:e16012. [Crossref] [PubMed]
- Boissière-Michot F, Lazennec G, Frugier H, et al. Characterization of an adaptive immune response in microsatellite-instable colorectal cancer. Oncoimmunology 2014;3:e29256. [Crossref] [PubMed]
- Samowitz WS, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology 2005;129:837-45. [Crossref] [PubMed]
- Tougeron D, Fauquembergue E, Rouquette A, et al. Tumour-infiltrating lymphocytes in colorectal cancers with microsatellite instability are correlated with the number and spectrum of frameshift mutations. Modern pathology: an official journal of the United States and Canadian Academy of Pathology Inc. 2009;22:1186-95.
- Zlobec I, Karamitopoulou E, Terracciano L, et al. TIA-1 cytotoxic granule-associated RNA binding protein improves the prognostic performance of CD8 in mismatch repair-proficient colorectal cancer. PLoS One 2010;5:e14282. [Crossref] [PubMed]
- Dolcetti R, Viel A, Doglioni C, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol 1999;154:1805-13. [Crossref] [PubMed]
- Guidoboni M, Gafà R, Viel A, Doglioni C, et al. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol 2001;159:297-304. [Crossref] [PubMed]
- Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350-6. [Crossref] [PubMed]
- Gavin PG, Colangelo LH, Fumagalli D, et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clin Cancer Res 2012;18:6531-41. [Crossref] [PubMed]
- Popovici V, Budinska E, Bosman FT, et al. Context-dependent interpretation of the prognostic value of BRAF and KRAS mutations in colorectal cancer. BMC Cancer 2013;13:439. [Crossref] [PubMed]
- Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011;117:4623-32. [Crossref] [PubMed]
- Lal N, Beggs AD, Willcox BE, et al. An immunogenomic stratification of colorectal cancer: Implications for development of targeted immunotherapy. Oncoimmunology 2015;4:e976052. [Crossref] [PubMed]
- Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nat Rev Cancer 2005;5:397-405. [Crossref] [PubMed]
- Lake RA, van der Most RG. A better way for a cancer cell to die. N Engl J Med 2006;354:2503-4. [Crossref] [PubMed]
- Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 2011;8:151-60. [Crossref] [PubMed]
- Correale P, Rotundo MS, Del Vecchio MT, et al. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother 2010;33:435-41. [Crossref] [PubMed]
- Oh DY, Venook AP, Fong L. On the Verge: Immunotherapy for Colorectal Carcinoma. J Natl Compr Canc Netw 2015;13:970-8. [PubMed]
- Droeser RA, Hirt C, Viehl CT, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 2013;49:2233-42. [Crossref] [PubMed]
- Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev 2014;23:2965-70. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Mellor JD, Brown MP, Irving HR, et al. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol 2013;6:1. [Crossref] [PubMed]
- Segal NH, Senzer N, Gada P, et al. Imprime PGG plus cetuximab therapy for advanced KRAS mutant colorectal cancer. ESMO 13th World Congress on Gastrointestinal Cancer, 2011. Barcelona, Spain: Oxford University Press.
- Strong JE, Coffey MC, Tang D, et al. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J 1998;17:3351-62. [Crossref] [PubMed]
- Riethmüller G, Holz E, Schlimok G, et al. Monoclonal antibody therapy for resected Dukes' C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol 1998;16:1788-94. [PubMed]
- Hartung G, Hofheinz RD, Dencausse Y, et al. Adjuvant therapy with edrecolomab versus observation in stage II colon cancer: a multicenter randomized phase III study. Onkologie 2005;28:347-50. [PubMed]
- Punt CJ, Nagy A, Douillard JY, et al. Edrecolomab alone or in combination with fluorouracil and folinic acid in the adjuvant treatment of stage III colon cancer: a randomised study. Lancet 2002;360:671-7. [Crossref] [PubMed]
- Halama N, Michel S, Kloor M, et al. The localization and density of immune cells in primary tumors of human metastatic colorectal cancer shows an association with response to chemotherapy. Cancer Immun 2009;9:1. [PubMed]


