An historical approach to the diagnostic biomarkers of acute coronary syndrome
Introduction
Despite the large efforts of the scientific community, cardiovascular disease (CVD) remains the main cause of death within industrialized nations as well as an increasing cause of death and morbidity in many developing countries (1,2). Between CVDs, the acute coronary syndrome (ACS) represents the most common cause of emergency hospital admission in Western countries (3,4), being associated with the highest mortality and morbidity (5).
Historically, ACS has included unstable angina (UA), non-ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI) (5).
Since ACS requires immediate hospital admission and the prognosis is directly associated with timely initiation of revascularization, missed, misdiagnosis or late diagnosis may have unfavorable clinical implications. The triage and management of patients with chest pain should be based on clear and well-defined pathways. Moreover, early ACS diagnosis reduces complications and long-term risk of recurrence, finally decreasing the economic burden posed on the health care system as a whole (6,7).
Despite the great efforts, the biochemical diagnostic approach to ACS remains one of most difficult and controversial medical challenges (8). Ideally, a biochemical marker of myocardial ischemia should have a considerable concentration in the myocardium, absence from non-myocardial tissue and normal serum, rapid release into the blood at the time of ischemia, a relationship to the extent of injury and persistence in the blood for a sufficient length of time to provide a diagnostic window. In addition, the test should be rapid, easy to perform and relatively inexpensive (9). At present, cardiac troponins are the only accepted biomarkers for diagnosing myocardial injury and acute myocardial infarction (AMI) (10). The increasing focus on biochemical markers during the last five decades [from amino transferases and lactate dehydrogenase (LDH) to cardiac troponins] (11), has led to the identification of a near-perfect biochemical marker (12,13).
1954: aspartate aminotransferase (AST)
In 1954, serum glutamic oxaloacetic transaminase (SGOT), now called AST, has been identified as the very first biochemical marker for diagnosis of AMI (14,15). The first method was originally based on paper chromatography and was hence extremely time-consuming. In the same year, a medical student, Arthur Karmen, developed a more rapid and practical spectrophotometric method to measure enzyme activity (16).
Years later, Henry et al. (17) improved the technique originally introduced by Karmen. In the reaction, the oxaloacetate produced by the transaminase serves as substrate for malate dehydrogenase by which it is reduced to malate in the presence of dihydronicotinamide-adenine dinucleotide (NADH), which is simultaneously oxidized. The reaction was monitored by a spectrophotometer as decrease in light absorption at 340 nm. The AST method was then standardized and adapted for use on many automatic analyzers (18).
AST increases in blood 3–4 hours after AMI, reaches the maximum value in blood in 15–28 hours and returns to normal values within 5 days (19). However, despite the high sensitivity for AMI, AST is a non-specific biomarker of cardiac tissue, wherein its activity can also increase in several other conditions including hepatic congestion secondary to congestive heart failure, myocarditis, electrical cardioversion, pericarditis, tachyarrhythmias, pulmonary embolism, and shock (20).
AST exists in human tissues as two distinct isoenzymes, one located in the cytoplasm (c-AST), and the other in mitochondria (m-AST), which differ in amino acid composition and immunochemical and kinetic properties (21). In particular, m-AST is infrequently enhanced after myocardial injury (22,23), increases later and apparently provides different biological information compared to c-AST (24). Rabkin et al. observed in their study performed on 15 AMI patients evaluated with invasive hemodynamic measurements, that m-AST correlated significantly with the hemodynamic assessment of left ventricular dysfunction of myocardial necrosis (23).
1955: lactate dehydrogenase (LDH)
Hill and Levi were the first to demonstrate the presence of LDH in human blood serum (25), and one year later Wróblewski and LaDue observed an increase in LDH activity in serum of patients with AMI (26,27). Ulmer et al. confirmed this observation in a study population of 22 AMI patients (28).
Since LDH is present in nearly all human tissues, LDH isoenzymes, either as a-hydroxybutyrate dehydrogenase (HBD) or lactate dehydrogenase isoenzyme 1 activities (LDH-1), were described as possible biomarkers of AMI (29,30) by providing more organ specificity than total LDH activity (19). Moreover, LDH-1 activity can be corrected for in vivo or in vitro hemolysis by measuring the ratio of LDH isoenzymes 1 and 2: the ratio is over 1.0 in AMI patients, whereas it remains below 1.0 in samples of patients with hemolysis (31).
LDH and its isoenzyme LDH-1 increase in blood 5–10 hours after AMI, reach the maximum value in blood in 60–144 hours and return to normal values in 12 days (19).
1960: creatine kinase (CK) total enzyme activity
The first spectrophotometric method for assessment of creatine phosphokinase was developed in 1955 by Oliver (32). Tanzer et al. then developed an enzymatic method for creatine and CK determination, characterized by greater specificity and sensitivity than the previous (33).
The assay for CK total enzyme activity was finally optimized by Rosalki in 1967 (34), by modifying the Kornberg ATP assay (35). Interestingly, Rosalki developed this method during a dinner and wrote it on the back of the menu card. This method required the addition of creatine phosphate, ADP, and a thiol, and the combination of all reagents in individual gelatin capsules. The modern fully automated clinical chemistry analyzers use now the same basic reagents, only slightly modified and optimized (36).
It was only in 1960 that the CK activity was shown to be a potential biomarker of cardiac muscle injury (37).
Since CK appears in blood 3–9 hours after an AMI, reaches the maximum value in blood in 10–20 hours and returns to normal values in approximately 72 hours (19), the sensitivity of this biomarker is very high when blood is collected early after the onset of disease. Sorensen reported a sensitivity of 98% in the AMI diagnosis when blood was collected within 72 hours after the onset of disease (38). Moreover, he also demonstrated that patients with high CK activity measurement in the third day had a worse prognosis.
Years later, it was shown that total CK activity may be related to the extent of myocardial infarction and prognosis (39,40). On the other hand, this biomarker is characterized by low specificity, since its activity increases considerably in liver, biliary tract, kidneys and skeletal muscles diseases.
1972: creatine kinase MB isoenzyme (CK-MB) activity
The enzyme CK is present in humans in three isoenzymes BB, MM and MB, the name of which originates from the various combination of the M (i.e., muscle) and brain (i.e., brain) isoforms. The CK-MB isoenzyme, which is normally undetectable or very low in the blood, increases in both heart and skeletal diseases by showing highest concentration in cardiac muscle (~22% of the total CK content of myocardium compared to ~1–3% in the skeletal muscle) (41). Several studies confirmed that CK-MB subforms provide a reliable and specific diagnosis with high accuracy in the first hours of onset of cardiac symptoms (42-44).
In 1972, Roe et al. developed a zone electrophoresis method for the identification and quantitation in serum or plasma of the CK-MB isoenzyme (45). Successively this biomarker was measured by anion-exchange column chromatography (46) and in 1976, Roberts et al. developed a radioimmunoassay (RIA) for CK isoenzymes (47).
The assays for measuring the enzymatic activity of CK-MB isoenzyme represented important advances, especially in terms of improved specificity (48).
In 1979, the World Health Organization (WHO) included in the criteria for AMI diagnosis the demonstration of typical rise or fall patterns of CK, CK-MB, LDH, or AST activities (49).
However, several preanalytical or analytical variables (i.e., prolonged storage or inadequate preservation, inhibitors or interference from other enzymes or drugs, pH and ionic concentrations used in the analyses and assay temperature) may influence the CK-MB activity (50-54). Moreover, the evidence that the activity of CK-MB can be considerably enhanced in many skeletal muscle disorders and that its concentration is characterized by a relatively slow release from the injured muscle cell, lead to way to additional research aimed to identified more reliable biomarkers.
1978: myoglobin
Myoglobin is a small (17.8 kDa) globular oxygen-carrying protein found in heart and striated skeletal muscle, with an almost identical amino acid sequence (55). It is a cytoplasmatic protein with a low molecular size and it is rapidly released after myocardial injury. It appears in blood 1–3 hours after AMI, reaches the maximum value in blood in 4–7 hours and returns to normal values after 1–1.5 days (19,56). However, because of rapid clearance from blood, myoglobin may “miss” late-presenting patients, and it is less cardiospecific than CK-MB (57).
Myoglobin concentration increases in skeletal muscular dystrophy, trauma, inflammation (myositis) or in presence of acute or chronic renal failure. Moreover, increased myoglobin levels can occur after muscle injections or strenuous exercise and in presence of various toxins and drugs (58).
The first method to detect myoglobin in serum was a RIA developed in 1978 (59,60). However this method was time-consuming and not useful for STAT analysis. Following the development of latex-enhanced immunoassays (61), myoglobin was introduced in the emergency department setting for identification of AMI (62). An automated non-isotopic immunoassay was also successively developed (63).
Despite myoglobin has been for long considered as the best marker for ruling out AMI in the emergency room from 3 to 6 hours after the onset of cardiac symptoms, the negative predictive value (NPV) reaches only 89%, at best (64).
On the other hand, since myoglobin is rapidly cleared from plasma after coronary reperfusion, it has been demonstrated that this biomarker may allow the earliest and best discrimination between reperfusion or no reperfusion in patients treated with intravenous thrombolytic therapy (65,66). Moreover, rapid kinetic of myoglobin is important for detecting re-infarction in patients with post-infarction angina when troponins are still elevated, or lese during revascularization procedures (67).
1985: CK-MB mass
The introduction of immunologic determination of CK-MB mass (i.e., protein concentration) was an important innovation, which virtually replaced the traditional enzymatic assay. The first “mass” immunoassay for CK-MB was developed in the 1985 (68) and was found to be much more sensitive than the measurement of enzymatic activity. One year later, Vaidya et al. developed a monoclonal antibody named “Conan MB” (in honor of a movie featuring the story of a barbarian warrior) directed against the CK-MB (69). This antibody was successively paired with an antibody to the B subunit of CK-MB. This two-site mass immunoassay is that currently used by all automated immunoassay instrumentation.
CK-MB mass measurement has the advantage to be more stable than the enzyme activity after storage and appears to be more sensitive, by increasing in plasma and serum more rapidly than CK or CK-MB activity (70,71). However, it is not sufficiently rapid when compared to myoglobin in the early diagnosis of AMI, mostly in the first 6 hours after symptom onset (72). As for the enzymatic activity, the mass value of CK-MB also increases in many conditions other than AMI (73).
In 1986, serum CK-MB mass measurement/total CK activity ratio was proposed to identify false-positive elevations of CK-MB arising from skeletal muscle (74). A ratio of less than 3 is consistent with a skeletal muscle source, while ratios greater than 5 are suggestive for a cardiac source. Ratios between 3 and 5 represent a gray zone.
In 1990, rapid enzyme immunoassays for direct mass measurement of CK-MB mass as µg/L were developed (75,76).
In the same year Delanghe et al. suggested that these immunoassays were less vulnerable to analytical interference and that measurement of CK-MB mass concentration is better suited for infarct sizing than measurement of catalytic activity (77).
1963: the discovery of troponins
The identification, purification, and characterization of troponins should be almost entirely attributed to Professor Setsuro Ebashi, whose landmark contributions in the early 1960s established the molecular basis of the Ca2+-regulation of muscle contraction. Its first contribution was the demonstration that calcium induced the contraction of actin and myosin filaments (78). Successively he showed that the muscle relaxing component known at that time as the “Marsh factor” was actually made by vesicles (79), later named sarcoplasmic reticulum, which contained an enzyme that used ATP energy to remove calcium from the medium by transporting it to their lumen (80). In 1963, he also demonstrated the existence of a third factor (besides myosin and actin) which conferred calcium sensitivity to actomyosin (81). This factor, tentatively named “native tropomyosin” because of its similarity with tropomyosin, was later shown to be a complex of tropomyosin and a new complex of proteins named troponins (82). He proved that this complex is the Ca2+-receptive site (83) and proposed the correct scheme for the molecular mechanism of regulation of contraction and relaxation (84). In the absence of Ca2+, the contractile interaction between myosin and actin is suppressed by troponin-tropomyosin complex. On increasing Ca2+ concentrations, this suppression is removed by the binding of Ca2+ to the troponin complex which activates the contraction (85).
Shortly after the discovery of the troponin complex, Ohtsuky, a graduate student working in Ebashi laboratory, showed, by an electron microscopic study, that it is distributed along the thin filament at regular intervals of about 400 A (86), thus leading to the construction of a model of thin filament as an ordered assembly of troponin, tropomyosin and actin (85).
1971: troponin isoforms
In 1971, Greaser and Gergely demonstrated that the troponin complex actually consists of three components which were named TnC, TnI, and TnT on the light of their specific properties: Ca2+ binding capacity (TnC), inhibition of ATPase activity (TnI) and tropomyosin binding respectively (TnT) (87). The existence of the three troponin components and the above nomenclature was generally accepted in 1972 in occasion of the Cold Spring Harbor Symposium on muscle, a meeting which would become a hallmark in the history of muscle study. In the follow ten years, many researcher groups became interested in the study of troponins and the knowledge about these proteins increased rapidly. Once the amino acid sequences of troponin isoforms was finally determined (88,89), it became possible to search for the regions of functional significance (90). Such findings were then followed by a number of studies of fluorescence resonance energy transfer, nucleic magnetic resonance and X-ray diffraction which finally led to the definition of the complete structure of troponin (91-93). In the meantime, gene expression studies showed that members of the TnC, TnI, and TnT gene families encode muscle-types specific isoforms differentially expressed in adult fast and slow skeletal muscles as well as in heart muscles. These include a fast skeletal and a slow skeletal-cardiac isoform of TnC (94-96), and a fast skeletal, a slow skeletal, and a cardiac isoform of both TnT and TnI (cTnT and cTnI). This exquisitely specific pattern of expression supported the use of cTnI and cTnT as biomarkers of cardiac injury.
Subsequent studies revealed that Mutations in the genes that encoding for two human cardiac Tn components, cTnI (TNNI3) and cTnT (TNNT2), are often responsible for cardiomyopathies (97-99).
1987: cTnI assays
In the 1980s, several research groups started to look at cardiac troponins as possibly specific cardiac biomarkers. Interest in TnI was prompted by the work of Cummins who developed the first RIA for the measurement of cTnI in serum in 1987 (100). This RIA methodology which was based on polyclonal rabbit antiserum, required two working days to be performed and had 10 ng/mL as the minimum detectable level. In his pioneer study Cummins showed that serum cTnI was elevated within 4 to 6 hours in patients with AMI, reached a mean peak level of 112 ng/mL (range, 20–550 ng/mL) at 18 hours, and remained above normal value for up to 8 days following myocardial injury. Three years later monoclonal antibodies directed against cTnI were described by two independent groups (101,102) one of which implemented an enzyme-linked immunoassay (ELISA) for quantification of serum cTnI. The assay developed by Bodor et al. had a detectable concentration of 1.9 µg/L and a working range of up to 100 µg/L. It required 3.5 hours to be performed (102). Such cTnI assay showed high specificity for cardiac injury even in the presence of acute muscle disease, chronic muscle disease, chronic renal failure, and after marathon running (103). During the following 20 years the cTnI immunoassay has been considerably optimized. Current generations of commercially available assays have an analytical sensitivity almost 100-fold higher (1 vs. 100 ng/L) than that of the experimental and commercial assays that were initially described. These assays were not fully standardized at this time and studies have documented substantial differences across methods (104,105). The main factors contributing to the quantitative differences between the cTnI methods include the lack of commutable reference material and difference in the antibody immunoreactivity as well as in the antigen used as calibrators (106). The analytical characteristics of cTn assays currently on market have been recently described by Jarolim (107).
1989: cTnT assays
The first generation immunoassay has been developed by Katus and colleagues in 1989. It was based on an ELISA with two antibodies: the capture antibody conjugated to biotin (M7) and the detection antibody conjugated to horseradish peroxidase (lBIO) (108). This assay, automatized in 1992 by its incorporation onto the ES-analysers (Boehringer Mannheim TM) (109), had two problems. The first was due to the assay formulation which comprised a completely cardiac-specific capture antibody (with <0.5% cross-reaction to skeletal muscle) and a detection antibody that was only 78% cardiospecific. The 20% cross-reactivity of the second antibody resulted in falsely TnT levels in patients with massive skeletal muscle damage (rhabdomyolysis). Such problem was soon overcome in 1997 with the introduction of the so-called ‘second generation’ TnT antibodies (M11.7 as capture antibody and M7 as detection antibody), which completely abolished the non-specific binding to skeletal TnT (110). With this second generation assay, the normal range of cTnT was between 0 and 0.1 µg/L. The limit of detection (LoD) and linearity of this assay were <0.05 and 12 µg/L, respectively.
The second problem was related to the platform. Although the test on the ES-analyser had been fully automated, it was characterized by a turn-around time of over 90 minutes with assays run daily, which was hence inadequate to fulfil requirements for emergency testing. This problem was also overcome by the introduction of the Elecsys TM analyzers, on which the turn-around time of the cTnT test was comprised between 9 (Elecsys 1010) and 18 (Elecsys 2010) min. At variance with the methodology of the ES analyser the Elecsys analyzers is based on electrochemiluminescence immunoassay (ECLIA) technology and uses a ruthenium labelled component instead of the horseradish peroxidase on the detection antibody (111).
In 1999, the ‘third generation’ troponin T assay has been introduced. The difference between the second and the third generation is the use of human recombinant cTnT for calibration (third generation) instead of bovine cTnT (second generation), which considerably improved the assay linearity (112). The fourth-generation cTnT assay, introduced in 2007, used fragment antigen-binding (FAB) of two cTnT-specific mouse monoclonal antibodies in a sandwich format. The antibodies recognized two epitopes located in the central part of the cTnT molecule. The fourth-generation cTnT assay has a LoD of 10 ng/L and a 10% coefficient of variation (CV) at 30 ng/L (113).
The new high-sensitivity cTnT (hs-cTnT) assay is a modification of the fourth-generation assay, which was implemented in 2010 (114). In this fifth generation assay the biotinylated capture antibody was not changed, whereas the detection antibody was genetically re-engineered into a mouse-human chimeric detection antibody to reduce the susceptibility to interference by heterophilic antibodies. The analytical sensitivity was improved by increasing the sample volume from 15 to 50 µL, increasing the ruthenium concentration of the detection antibody, and lowering the background signal through buffer optimization. As a result, the analytic performance of the hs-cTnT assay had been significantly improved; the LoD was 5 ng/L, the 99th percentile cutoff point was 14 ng/L, and the CV was 10% at 13 ng/L.
Due to patent issues, cTnT assays are only available from one manufacturer (Roche Diagnostic). Therefore, in contrast to cTnI, standardization of the cTnT assay is not seen as a major problem. The only inconvenience is the current coexistence of the less sensitive fourth generation assay in the USA and the hs-cTnT assay in most other countries, since the hs-cTnT has not been licensed for use by the FDA so far.
2012: diagnostic value of cTn in AMI
According to the international consensus and task force definition of AMI established in 2012 (115), the diagnosis of AMI is based mainly on evidence of myocardial ischemia, along with an elevated value of cardiac biomarkers above the 99th percentile and demonstration of an increase or decrease over time.
The continuous improvement of the analytical sensitivity and assay precision at the low measuring range of cTn assays has ultimately led to the development of the so-called “hs” cTn assays which finally satisfy this criterion. In order to label a cTn assay as “hs”, the IFCC task force suggested that cTn should be measurable in more than 50% of healthy subjects, and preferably in more than 95% (116). The term ultra-sensitive is conventionally reserved to cTnI assay capable to quantify cTn at levels below the lowest concentrations seen in healthy subjects (117). The interest in this additional sensitivity goes beyond the management of patients with suspected MI and is limited to novel application of cTn assays such as measuring changes in cTn levels after exercise stress testing or after cardiotoxic chemotherapy (118). Nowadays, hs-cTn assays are considered the biomarkers of choice in the early diagnosis of AMI being able to detect cTn release at an earlier time point than the previous generations of cTn assays, especially in patients with a recent onset of chest pain (119-122). Most patients with an AMI, can be reliably identified within 3 h after admission, with nearly 100% sensitivity and 100% NPV using a hs-cTn assay, which indicates that observation time in the emergency department may be reduced for rule out of AMI (12). However, in patients with 3 h values unchanged, but in whom pre-test likelihood of AMI is high, additional subsequent sampling (e.g., at 2 or 3 h) may still be advisable.
As predictable, the improved sensitivity of the new generations immunoassays came along with a decreased specificity for AMI. Measurable troponin values can now be found in several non-ischemic cardiac conditions, including, among the other, atrial fibrillation, hypertension, renal and liver disorders, acute or chronic pulmonary disease (123) and even severe allergic reactions (124). Therefore, careful clinical assessment, serial testing and thoughtful differentiation are required to separate AMI from other acute and chronic disorders which can be associated with low-level and less harmful myocardial injury (125).
Conclusions
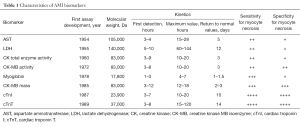
The relatively long history of AMI diagnostics has been marked by many milestones (Table 1). After more than 60 years of research we have now come to a point when hs-cTN immunoassays should be considered as “the best there is”. But, with ongoing technological advances and increasing knowledge of the pathophysiology of myocardial ischemia, it seems premature to conclude that hs-cTn will also be “the best there will ever be”. Many questions remain unanswered, mainly concerning the optima cut-offs and timing of serial sampling. Hopefully, further studies will help refine the clinical use of hs-cTn immunoassays in myocardial injury.
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bhatnagar P, Wickramasinghe K, Williams J, et al. The epidemiology of cardiovascular disease in the UK 2014. Heart 2015;101:1182-9. [Crossref] [PubMed]
- Wilmot KA, O'Flaherty M, Capewell S, et al. Coronary heart disease mortality declines in the united states from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation 2015;132:997-1002. [Crossref] [PubMed]
- Makam AN, Nguyen OK. Use of cardiac biomarker testing in the emergency department. JAMA Intern Med 2015;175:67-75. [Crossref] [PubMed]
- Storrow AB, Gibler WB. Chest pain centers: diagnosis of acute coronary syndromes. Ann Emerg Med 2000;35:449-61. [Crossref] [PubMed]
- Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc 2009;84:917-38. [Crossref] [PubMed]
- Wenaweser P, Windecker S. Acute coronary syndromes: management and secondary prevention. Herz 2008;33:25-37. [Crossref] [PubMed]
- Goodacre S, Thokala P, Carroll C, et al. Systematic review, meta-analysis and economic modelling of diagnostic strategies for suspected acute coronary syndrome. Health Technol Assess 2013;17:v-vi, 1-188. [Crossref] [PubMed]
- Rosalki SB, Roberts R, Katus HA, et al. Cardiac biomarkers for detection of myocardial infarction: perspectives from fast to present. Clin Chem 2004;50:2205-13. [Crossref] [PubMed]
- Lippi G, Montagnana M, Salvagno GL, et al. Potential value for new diagnostic markers in the early recognition of acute coronary syndromes. CJEM 2006;8:27-31. [PubMed]
- Gamble JH, Carlton EW, Orr WP, et al. High-sensitivity cardiac troponins: no more 'negatives'. Expert Rev Cardiovasc Ther 2013;11:1129-39. [Crossref] [PubMed]
- Wu AH. Cardiac markers: from enzymes to proteins, diagnosis to prognosis, laboratory to bedside. Ann Clin Lab Sci 1999;29:18-23. [PubMed]
- Lippi G. Biomarkers: Novel troponin immunoassay for early ACS rule-out. Nat Rev Cardiol 2016;13:9-10. [Crossref] [PubMed]
- Dolci A, Panteghini M. The exciting story of cardiac biomarkers: from retrospective detection to gold diagnostic standard for acute myocardial infarction and more. Clin Chim Acta 2006;369:179-87. [Crossref] [PubMed]
- Ladue JS, Wrŏblewski F, Karmen A. Serum glutamic oxaloacetic transaminase activity in human acute transmural myocardial infarction. Science 1954;120:497-9. [Crossref] [PubMed]
- Karmen A, Wroblewski F, LaDue JS. Transaminase activity in human blood. J Clin Invest 1955;34:126-31. [Crossref] [PubMed]
- Karmen A. A note on the spectrophotometric assay of glutamic-oxaloacetic transaminase in human blood serum. J Clin Invest 1955;34:131-3. [PubMed]
- Henry RJ, Chiamori N, Golub OJ, et al. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase, and lactic acid dehydrogenase. Am J Clin Pathol 1960;34:381-98. [PubMed]
- Wilkinson JH, Baron DN, Moss DW, et al. Standardization of clinical enzyme assays: a reference method for aspartate and alanine transaminases. J Clin Pathol 1972;25:940-4. [Crossref] [PubMed]
- Penttilä I, Penttilä K, Rantanen T. Laboratory diagnosis of patients with acute chest pain. Clin Chem Lab Med 2000;38:187-97. [Crossref] [PubMed]
- Johnston CC, Bolton EC. Cardiac enzymes. Ann Emerg Med 1982;11:27-35. [Crossref] [PubMed]
- Boyde TR, Kwong EM. Aspartate aminotransferase isoenzymes--differential kinetic assay in serum. Clin Chim Acta 1983;128:95-102. [Crossref] [PubMed]
- Boyde TR. Serum levels of the mitochondrial isoenzyme of aspartate aminotransferase in myocardial infarction and muscular dystrophy. Enzymol Biol Clin (Basel) 1968;9:385-92. [PubMed]
- Rabkin SW, Desjardins P. Mitochondrial and cytoplasmatic isoenzymes of aspartate aminotransferase in sera of patients after myocardial infarction. Clin Chim Acta 1984;138:245-57. [Crossref] [PubMed]
- Panteghini M, Pagani F, Cuccia C. Activity of serum aspartate aminotransferase isoenzymes in patients with acute myocardial infarction. Clin Chem 1987;33:67-71. [PubMed]
- Hill BR, Levi C. Elevation of a serum component in neoplastic disease. Cancer Res 1954;14:513-5. [PubMed]
- Wroblewski F, LaDue JS. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med 1955;90:210-3. [Crossref] [PubMed]
- Wróblewski F, Ruegsegger P, LaDue JS. Serum lactic dehydrogenase activity in acute transmural myocardial infarction. Science 1956;123:1122-3. [Crossref] [PubMed]
- Ulmer DD, Vallee BL, Wacker WE. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med 1956;255:450-6. [PubMed]
- Wróblewski F, Ross C, Gregory K. Isoenzymes and myocardial infarction. N Engl J Med 1960;263:531-6. [Crossref] [PubMed]
- Konttinen A. Hydroxybutyrate dehydrogenase in detection of myocardial infarction. Lancet 1961;2:556-8. [Crossref] [PubMed]
- Galbraith LV, Leung FY, Jablonsky G, et al. Time related changes in the diagnostic utility of total lactate dehydrogenase, lactate dehydrogenase isoenzyme-1, and two lactate dehydrogenase isoenzyme-1 ratios in serum after myocardial infarction. Clin Chem 1990;36:1317-22. [PubMed]
- Oliver IT. A spectrophotometric method for the determination of creatine phosphokinase and myoldnase. Biochem J 1955;61:116-22. [Crossref] [PubMed]
- Tanzer ML, Gilvarg C. Creatine and creatine measurement. J Biol Chem 1959;234:3201-4. [PubMed]
- Rosalki SB. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med 1967;69:696-705. [PubMed]
- Kornberg A. Reversible enzymatic synthesis of diphosphopyridine nucleotide and inorganic pyrophosphate. J Biol Chem 1948;176:1475. [PubMed]
- Schumann G, Bonora R, Ceriotti F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 2. Reference procedure for the measurement of catalytic concentration of creatine kinase. Clin Chem Lab Med 2002;40:635-42. [PubMed]
- Dreyfus JC, Schapira G, Rasnais J, et al. Serum creatine kinase in the diagnosis of myocardial infarct. Rev Fr Etud Clin Biol 1960;5:386-7. [PubMed]
- Sorensen NS. Creatine phosphokinase in the diagnosis of myocardial infarction. Acta Med Scand 1963;174:725-34. [Crossref] [PubMed]
- Shell WE, Kjekshus JK, Sobel BE. Quantitative assessment of the extent of myocardial infarction in the conscious dog by means of analysis of serial changes in serum creatine phosphokinase activity. J Clin Invest 1971;50:2614-25. [Crossref] [PubMed]
- Sobel BE, Bresnahan GF, Shell WE, et al. Estimation of infarct size in man and its relation to prognosis. Circulation 1972;46:640-8. [Crossref] [PubMed]
- Panteghini M. Enzyme and muscle diseases. Curr Opin Rheumatol 1995;7:469-74. [Crossref] [PubMed]
- Puleo PR, Guadagno PA, Roberts R, et al. Early diagnosis of acute myocardial infarction based on assay for subforms of creatine kinase-MB. Circulation 1990;82:759-64. [Crossref] [PubMed]
- Wu AH, Wang XM, Gornet TG, et al. Creatine kinase MB isoforms in patients with skeletal muscle injury: ramifications for early detection of acute myocardial infarction. Clin Chem 1992;38:2396-400. [PubMed]
- Zimmerman J, From R, Meyer D, et al. Diagnostic marker cooperative study for the diagnosis of myocardial infarction. Circulation 1999;99:1671-7. [Crossref] [PubMed]
- Roe CR, Limbird LE, Wagner GS, et al. Combined isoenzyme analysis in the diagnosis of myocardial injury: Application of electrophoretic methods for the detection and quantitation of the creatine phosphokinase MB isoenzyme. J Lab Clin Med 1972;80:577. [PubMed]
- Mercer DW. Separation of tissue and serum creatine kinase isoenzymes by ion-exchange column chromatography. Clin Chem 1974;20:36-40. [PubMed]
- Roberts R, Sobel BE, Parker CW. Radioimmunoassay for creatine kinase isoenzymes. Science 1976;194:855-7. [Crossref] [PubMed]
- Bruns DE, Chitwood J, Koller K, et al. Creatine kinase-MB activity: clinical and laboratory studies of specific immunochemical technique with optimized enzymatic assay. Ann Clin Lab Sci 1983;13:59-66. [PubMed]
- World Health Organization. Report of the Joint International Society and Federation of Cardiology/World Health Organization Task Force on Standardization of Clinical Nomenclature. Nomenclature and criteria for diagnosis of ischemic heart disease. Circulation 1979;59:607-9. [Crossref] [PubMed]
- Szasz G, Gruber W, Bernt E. Creatine kinase in serum: 1. Determination of optimum reaction conditions. Clin Chem 1976;22:650-6. [PubMed]
- Young DS, Pestaner LC, Gibberman V. Effects of drugs on clinical laboratory tests. Clin Chem 1975;21:1D-432D. [PubMed]
- Perry B, Doumas B, Jendrzejczak B. Effect of light and temperatare on the stability of creatine kunase in human sera and controls. Clin Chem 1979;25:625-8. [PubMed]
- Dalal FR, Chilley J Jr, Winsten S. A study of the problems of inactivation of creatine kinase in serum. Clin Chem 1972;18:330-4. [PubMed]
- Szasz G, Gerhardt W, Gruber W. Creatine kinase in serum: 5. Effect of thiols on isoenzyme activity during storage at various temperatures. Clin Chem 1978;24:1557-63. [PubMed]
- Adams JE, Abendschein DR, Jaffe AS. Biochemical markers of myocardial injury. Circulation 1993;88:750-63. [Crossref] [PubMed]
- Ohman EM, Casey C, Bengtson JR, et al. Early detection of acute myocardial infarction: additional diagnostic information from serum concentrations of myoglobin in patients without ST elevation. Br Heart J 1990;63:335-8. [Crossref] [PubMed]
- French JK, White HD. Clinical implications of the new definition of myocardial infarction. Heart 2004;90:99-106. [Crossref] [PubMed]
- Plebani M, Zaninotto M. Diagnostic strategies using myoglobin measurement in myocardial infarction. Clin Chim Acta 1998;272:69-77. [Crossref] [PubMed]
- Gilkeson G, Stone MJ, Waterman M, et al. Detection of myoglobin by radioimmunoassay in human sera: its usefulness and limitations as an emergency room screening test for acute myocardial infarction. Am Heart J 1978;95:70-7. [Crossref] [PubMed]
- Varki AP, Roby DS, Watts H, et al. Serum myoglobin in acute myocardial infarction: a clinical study and review of the literature. Am Heart J 1978;96:680-8. [Crossref] [PubMed]
- Schultz A, Larsen CE, Kristensen SD, et al. Serum myoglobin measured by latex agglutination: rapid test for exclusion of acute myocardial infarction. Am Heart J 1986;112:609-10. [Crossref] [PubMed]
- Gibler WB, Gibler CD, Weinshenker E, et al. Myoglobin as an early indicator of acute myocardial infarction. Ann Emerg Med 1987;16:851-6. [Crossref] [PubMed]
- Wu AHB, Laios I, Green S, et al. Immunoassays for serum and urine myoglobin: myoglobin clearance assessed as a risk factor for acute renal failure. Clin Chem 1994;40:796-802. [PubMed]
- de Winter RJ, Koster RW, Sturk A, et al. Value of myoglobin, troponin T, and CK-MB mass in ruling out an acute myocardial infarction in the emergency room. Circulation 1995;92:3401-7. [Crossref] [PubMed]
- Katus HA, Diederich KW, Scheffold T, et al. Non-invasive assessment of infarct reperfusion: the predictive power of the time to peak value of myoglobin, CKMB, and CK in serum. Eur Heart J 1988;9:619-24. [PubMed]
- Ellis AK, Little T, Masud AR, et al. Early noninvasive detection of successful reperfusion in patients with acute myocardial infarction. Circulation 1988;78:1352-7. [Crossref] [PubMed]
- Grenadier E, Keidar S, Kahana L, et al. The roles of serum myoglobin, total CPK, and CK-MB isoenzyme in the acute phase of myocardial infarction. Am Heart J 1983;105:408-16. [Crossref] [PubMed]
- Chan DW, Taylor E, Frye T, et al. Immunoenzymetric assay for creatine kinase MB with subunitspecific monoclonal antibodies compared with an immunochemical method and electrophoresis. Clin Chem 1985;31:465-9. [PubMed]
- Vaidya HC, Maynard Y, Dietzler DN, et al. Direct measurement of creatine kinase-MB activity in serum after extraction with a monoclonal antibody specific to the MB isoenzyme. Clin Chem 1986;32:657-63. [PubMed]
- Murthy VV, Karmen A. Activity concentration and mass concentration (monoclonal antibody immunoenzymometric method) compared for creatine kinase MB isoenzyme in serum. Clin Chem 1986;32:1956-9. [PubMed]
- Bakker AJ, Gorgels JP, van Vlies B, et al. The mass concentrations of serum troponin T and creatine kinase-MB are elevated before creatine kinase and creatine kinase–MB activities in acute myocardial infarction. Eur J Clin Chem Clin Biochem 1993;31:715-24. [PubMed]
- Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 2000;21:1502-13. [Crossref] [PubMed]
- Pierce GF, Jaffe AS. Increased creatine kinase MB in the absence of acute myocardial infarction. Clin Chem 1986;32:2044-51. [PubMed]
- el Allaf M, Chapelle JP, el Allaf D, et al. Differentiating muscle damage from myocardial injury by means of the serum creatine kinase (CK) isoenzyme MB mass measurement/total CK activity ratio. Clin Chem 1986;32:291-5. [PubMed]
- Brandt DR, Gates RC, Eng KK, et al. Quantifying the MB isoenzyme of creatine kinase with the Abbott IMx immunoassay analyzer. Clin Chem 1990;36:375-8. [PubMed]
- Jørgensen PJ, Hørder M, Selmer J, et al. Analytical evaluation of a sensitive enzyme immunoassay for the determinations of creatine kinase isoenzyme MB. Clin Chem 1990;36:1502-5. [PubMed]
- Delanghe JR, De Mol AM, De Buyzere ML, et al. Mass concentration and activity concentration of creatine kinase isoenzyme MB compared in serum after acute myocardial infarction. Clin Chem 1990;36:149-53. [PubMed]
- Ebashi S. Calcium binding and relaxation in the actomyosin system. J Biochem 1960;48:150-1.
- Ebashi S. Calcium binding activity of vesicular relaxing factor. J Chir (Paris) 1961;82:236-44. [PubMed]
- Ebashi S, Lipmann F. Adenosine triphosphate-linked concentration of calcium ions in a particulate fraction of rabbit muscle. J Cell Biol 1962;14:389-400. [Crossref] [PubMed]
- Ebashi S. Third component participating in the superprecipitation of ‘natural actomyosin’. Nature 1963;200:1010. [Crossref] [PubMed]
- Ebashi S, Kodama A. A new protein factor promoting aggregation of tropomyosin. J Biochem 1965;58:107-8. [PubMed]
- Ebashi S, Ebashi F, Kodama A. Troponin as the Ca++-receptive protein in the contractile system. J Biochem 1967;62:137-8. [PubMed]
- Ebashi S, Endo M. Calcium and muscle contraction. Prog Biophys Mol Biol 1968;18:123-83. [Crossref] [PubMed]
- Ebashi S, Endo M, Ohtsuki I. Control of muscle contraction. Q Rev Biophys 1969;2:351-84. [Crossref] [PubMed]
- Otsuki I, Masaki T, Nonomura Y, et al. Periodic distribution of troponin along the thin filament. J Biochem 1967;61:817-9. [PubMed]
- Greaser ML, Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem 1971;246:4226-33. [PubMed]
- Collins JH, Potter JD, Horn MJ, et al. The amino acid sequence of rabbit skeletal muscle troponin C: gene replication and homology with calcium-binding proteins from carp and hake muscle. FEBS Lett 1973;36:268-72. [Crossref] [PubMed]
- Wilkinson JM, Grand RJ. Comparison of amino acid sequence of troponin I from different striated muscles. Nature 1978;271:31-5. [Crossref] [PubMed]
- Leavis PC, Gowell E, Tao T. Fluorescence lifetime and acrylamide quenching studies of the interactions between troponin subunits. Biochemistry 1984;23:4156-61. [Crossref] [PubMed]
- Herzberg O, James MN. Structure of the calcium regulatory muscle protein troponin-C at 2.8 A resolution. Nature 1985;313:653-9. [Crossref] [PubMed]
- Hitchcock-De Gregori SE. Study of the structure of troponin-I by measuring the relative reactivities of lysines with acetic anhydride. J Biol Chem 1982;257:7372-80. [PubMed]
- Vassylyev DG, Takeda S, Wakatsuki S, et al. Crystal structure of troponin C in complex with troponin I fragment at 2.3-A resolution. Proc Natl Acad Sci U S A 1998;95:4847-52. [Crossref] [PubMed]
- Dhoot GK, Frearson N, Perry SV. Polymorphic forms of troponin T and troponin C and their localization in striated muscle cell types. Exp Cell Res 1979;122:339-50. [Crossref] [PubMed]
- Mikawa T, Takeda S, Shimizn T, et al. Gene expression of myofibrillar proteins in single muscle fibers of adult chicken: micro two dimensional gel electrophoretic analysis. J Biochem 1981;89:1951-62. [PubMed]
- Bucher EA, Maisonpierre PC, Konieczny SF, et al. Expression of the troponin complex genes: transcriptional coactivation during myoblast differentiation and independent control in heart and skeletal muscles. Mol Cell Biol 1988;8:4134-42. [Crossref] [PubMed]
- Fatkin D, Graham RM. Molecular mechanism of inherited cardiomyopathies. Physiol Rev 2002;82:945-80. [Crossref] [PubMed]
- Harada K, Potter JD. Familial hypertrophic cardiomyopathy mutations from different functional regions of troponin T result in different effects on the pH- and Ca2+-sensitivity of cardiac muscle contraction. J Biol Chem 2004;279:14488-95. [Crossref] [PubMed]
- Lippi G, Cervellin G. Genetic polymorphisms of human cardiac troponins as an unrecognized challenge for diagnosing myocardial injury. Int J Cardiol 2014;171:467-70. [Crossref] [PubMed]
- Cummins B, Auckland ML, Cummins P. Cardiac-specific troponin-I radioimmunoassay in the diagnosis of acute myocardial infarction. Am Heart J 1987;113:1333-44. [Crossref] [PubMed]
- Larue C, Defacque-Lacquement H, Calzolari C, et al. New monoclonal antibodies as probes for human cardiac troponin I: Epitopic analysis with synthetic peptides. Mol Immunol 1992;29:271-8. [Crossref] [PubMed]
- Bodor GS, Porter S, Landt Y, et al. Development of monoclonal antibodies for an assay of cardiac troponin I and preliminary results in suspected cases of myocardial infarction. Clin Chem 1992;38:2203-14. [PubMed]
- Adams JE III, Bodor GS, Dávila-Román VG, et al. Cardiac troponin I: a marker with high specificity for cardiac injury. Circulation 1993;88:101-6. [Crossref] [PubMed]
- Lippi G, Salvagno GL, Da Rin G, et al. Harmonization of contemporary-sensitive troponin I immunoassays: calibration may only be a part of the problem. Riv Ital Med Lab 2014;10:108-14.
- Salvagno GL, Giavarina D, Meneghello M, et al. Multicenter Comparison of Four Contemporary Sensitive Troponin Immunoassays. J Med Biochem 2014;33:271-7. [Crossref]
- Apple FS. Counterpoint: Standardization of cardiac troponin I assays will not occur in my lifetime. Clin Chem 2012;58:169-71. [Crossref] [PubMed]
- Jarolim P. High sensitivity cardiac troponin assays in the clinical laboratories. Clin Chem Lab Med. 2015;53:635-52. [Crossref] [PubMed]
- Katus HA, Remppis A, Looser S, et al. Enzyme-linked immunoassay of cardiac troponin T for the detection of acute myocardial infarction in patients. J Mol Cell Cardiol 1989;21:1349-53. [Crossref] [PubMed]
- Katus HA, Looser S, Hallermeyer K, et al. Development and in vitro characterization of a new immunoassay of cardiac troponin T. Clin Chem 1992;38:386-93. [PubMed]
- Müller-Bardorff M, Hallermeyer K, Schroder A, et al. Improved Troponin T ELISA specific for cardiac Troponin T isoform: assay development and analytical and clinical validation. Clin Chem 1997;43:458-66. [PubMed]
- Hoyle NR, Eckert B, Krais S. Electrochemiluminescence: leading-edge technology for automated immunoassay analyte detection. Clin Chem 1996;42:1576-8.
- Hallermayer K, Klenner D, Vogel R. Use of recombinant human cardiac Troponin T for standardization of third generation Troponin T methods. Scand J Clin Lab Invest Suppl 1999;230:128-31. [Crossref] [PubMed]
- Hermsen D, Apple F, Garcia-Beltran L, et al. Results from a multicenter evaluation of the 4th generation Elecsys Troponin T assay. Clin Lab 2007;53:1-9. [PubMed]
- Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010;56:254-61. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581-98. [Crossref] [PubMed]
- Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54-61. [Crossref] [PubMed]
- Lippi G. The mystifying nomenclature of cardiac troponin immunoassays. Scand J Clin Lab Invest 2014;74:273-7. [Crossref] [PubMed]
- Roziakova L, Bojtarova E, Mistrik M, et al. Serial measurements of cardiac biomarkers in patients after allogeneic hematopoietic stem cell transplantation. J Exp Clin Cancer Res 2012;31:13. [Crossref] [PubMed]
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361:858-67. [Crossref] [PubMed]
- Weber M, Bazzino O, Navarro Estrada JL, et al. Improved diagnostic and prognostic performance of a new high-sensitive troponin T assay in patients with acute coronary syndrome. Am Heart J 2011;162:81-8. [Crossref] [PubMed]
- Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med 2009;361:868-77. [Crossref] [PubMed]
- Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011;306:2684-93. [Crossref] [PubMed]
- Lippi G. Biomarkers of myocardial ischemia in the emergency room: cardiospecific troponin and beyond. Eur J Intern Med 2013;24:97-9. [Crossref] [PubMed]
- Lippi G, Buonocore R, Schirosa F, et al. Cardiac troponin I is increased in patients admitted to the emergency department with severe allergic reactions. A case-control study. Int J Cardiol 2015;194:68-9. [Crossref] [PubMed]
- Agewall S, Giannitsis E, Jernberg T, et al. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J 2011;32:404-11. [Crossref] [PubMed]


