The worst of both worlds-combined pulmonary fibrosis and emphysema syndrome
Introduction
Rarely, we will see patient in our practice who might appear to have a combination of two different disease belonging to opposing ends of a spectrum of clinical features. Combined pulmonary fibrosis and emphysema syndrome (CPFE) is one of such rare conditions where the physician encounters a mix of clinical features which are thought to be inconsonant. These patients have a variable mix of pulmonary fibrosis and emphysema and a very different course compared to patients with either disease alone.
Case presentation
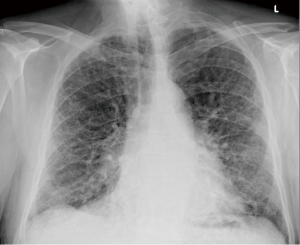
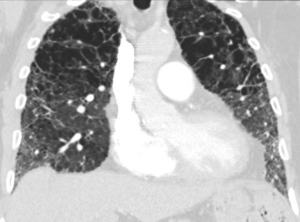
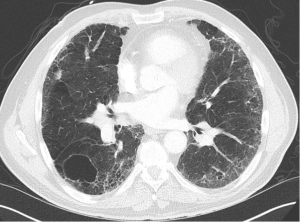
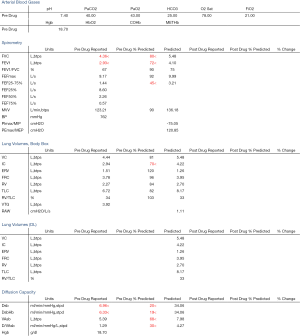
We present a case of a severely dyspneic 63-year-old man with features of both emphysema and pulmonary fibrosis. He was diagnosed with emphysema four years back and has been using two liters of oxygen till eight months back when he started getting more short of breath and had a rapid increase in his oxygen requirement. He is an ex-smoker with a 30-pack year smoking history and quit smoking seven years back. He is a mechanical engineer by profession, has a history of exposure to asbestos but denied any exposure to birds, chemicals or molds. He does not have any pets at home or any significant travel history. He denied any fevers, chills, hemoptysis, or chest pain. On evaluation, he was hypoxic on 6 L of oxygen and was desaturating to the high 80 s even with mild exertion. The patient was afebrile, normotensive, sitting up in a chair at the time of initial assessment. He had a regular tachycardia of 101 beats/min and a respiratory rate of 28 breaths/min. Auscultation of the lungs revealed basal inspiratory crepitation with prolonged expiratory wheezes scattered throughout both lung fields. He had grade III clubbing bilaterally. Examination of other organ systems was unrevealing. Initial chest X-ray revealed bilateral diffuse interstitial fibrosis more prominent in the periphery of the lungs. Bilateral apical pleural thickening was also noted (Figure 1). CT of the chest demonstrated extensive ground glass opacities with centrilobular emphysematous changes at the apices (Figure 2). Bilateral lower lobe predominant fibrosis with honeycombing and traction bronchiectasis were also noted (Figure 2). A thick walled cystic lesion (TWCL) was present in the lower lobe (Figure 3). Pulmonary function tests (PFT) demonstrated mild obstruction with severely reduced diffusion capacity for oxygen (DLCO) (Figure 4). His echocardiogram revealed ejection fraction of 50–55%, right ventricular systolic pressure of 35–40 mmHg with evidence of left ventricular diastolic dysfunction. Laboratory studies demonstrated a leukocyte count of 10.2 thousand/mL with 73% granulocytes, hemoglobin of 17.4 gm/dL, and a normal metabolic panel. Immunological work up was unrevealing with a negative antinuclear, anti-double stranded DNA, anti-neutrophil cytoplasmic, rheumatoid factor, anti-SSA/Roro and anti-La/SSB, anti-scleroderma, anti-cyclic citrullinated peptide and anti-Jo 1 antibodies.
Discussion
There are two clinical scenarios that could explain this unusual mix of findings. This could either represent preexisting emphysema with superimposed pulmonary fibrosis or vice-versa i.e., preexisting pulmonary fibrosis with superimposed emphysema. There also exists a third possibility, one which fits best with this particular clinical presentation and imaging features, appropriately termed the syndrome of CPFE. This syndrome was first described by Cottin et al. in 2005 (1). In the absence of a consensus definition, we can use certain clinical characteristics to help us identify this syndrome (2):
- Male (9:1; M:F);
- Heavy smoker;
- Severely symptomatic;
- Characteristic PFT with isolated reduction in DLCO;
- High incidence of pulmonary hypertension (PH);
- Have a high incidence of lung cancer.
There is a lack of clear epidemiological data to clarify the prevalence of this syndrome. A review of IPF literature indicates that between 8% to 51% of CT scans from patients with idiopathic pulmonary fibrosis (IPF) demonstrates some degree of emphysema (3,4). Similarly, review of lung imaging data among those diagnosed with emphysema shows that 4.4% to 8% of patients have some degree of lung fibrosis (5,6). The importance of identifying this condition lies in the fact that up to 35% of patients with IPF may actually have CPFE and thus may have a different clinical course than patients with IPF alone (2). These patients depend on compensatory hypoxic vasoconstriction to modify their ventilation/perfusion mismatch and attempt to preserve normoxemia which puts them at risk of having a paradoxical hypoxemic response to commonly utilized pulmonary vasodilator therapies with prostacyclin analogues, endothelin receptor antagonists and phosphodiesterase inhibitors aimed at treating PH in such patients (7). Patients with CPFE are at a high risk of developing lung cancer and require annual surveillance CT scans (8) and also need to be referred for lung transplant earlier than somebody with similar degree of emphysema or fibrosis alone. With a normal to near normal FEV1, their BODE (Body-mass index, airflow Obstruction, Dyspnea, and Exercise) indexes are falsely low and do not accurately reflect the severity of their condition. Thus BODE scores are not an accurate way of assessing candidacy for lung transplant among such patients. Quite clearly, those with CPFE have a disease that has a different clinical course from those patients with IPF and COPD and hence, trials on treatment for COPD and IPF have always excluded patients with CPFE.
The identification of CPFE requires the physician to be familiar with radiological findings in both interstitial lung diseases and emphysema. A mix of all three types of emphysema are noted in the upper lobes with centrilobular emphysema (97%) being the most common followed by paraseptal emphysema (93%) and bullae (54%) (2). Paraseptal emphysema is seen much more frequently among patients with CPFE than those with emphysema alone. This provides support for the hypothesis that paraseptal and centrilobular emphysema have different inflammatory signature, different genes and result from different responses to smoke inhalation. Thick-walled cystic lesions (TWCL) (Figure 3) have been increasingly recognized as unique radiological and pathological features of CPFE (9). Enlargement of TWCLs are thought to reflect the worsening of interstitial fibrosis and progression of the disease. Autopsy studies have revealed that radiological and pathological TWCLs were observed in 72.7% of the CPFE patients, but not in any patient with either IPF or emphysema alone (10). Features of pulmonary fibrosis include honeycombing which is the most common feature (75.6–95%) followed by reticulation (84.4–87%) and traction bronchiectasis (40–69%) (2,11). The degree of emphysema based on CT emphysema scores are reported to be similar to those with mild to moderate emphysema but lower than those with severe emphysema (12,13).
CPFE is thought to be the result of a poorly-understood interaction between environmental and genetic factors, among which the best recognized is cigarette smoke. A high frequency of fibrosis has been observed in pieces of lung tissue removed for tumor surgery from smokers. These patients showed no clinical evidence of interstitial lung disease (14). Kaolinite or aluminum silicate in cigarette smoke is reported to lead to macrophage accumulation after chronic inhalation and trigger a series of pathophysiological events leading to respiratory bronchiolitis-interstitial lung disease (RB-ILD) and emphysema in animal models (15). Other occupational factors such as welding (16) or exposure to talc (17) have been reported to be associated with CPFE. CPFE syndrome has been described in a series of patients with connective tissue diseases (CTD), mainly among smokers or former smokers with rheumatoid arthritis and systemic sclerosis. Mutation in the following genes has been reported to result in the development of CPFE.
- Surfactant protein C (SFTPC) gene (18);
- ABCA3 mutation (19);
- Mutations in the essential telomerase genes (hTERT or hTR is a risk factors for pulmonary fibrosis in up to one-fifth of familial cases). Association between CPFE and mutation in the hTERT gene has been reported in several individuals of a family with the CPFE phenotype (20).
Management
CPFE syndrome is associated with poor survival statistics, worse than those with pulmonary fibrosis or emphysema alone. A five-year survival of only 35–80% and a median survival ranging between 2.1 to 8.5 years have been reported (1,2). The major causes of death in CPFE are chronic respiratory failure, PH, acute exacerbation and lung cancer. One of the reasons for this low survival rates is the lack of clear management guidelines for such patients combined with the fact that these patients develop life threatening complications earlier than those with pulmonary fibrosis or emphysema alone. Patients with CPFE are excluded from trials on IPF and COPD, and as a result, the usefulness of newer therapies in such patients is understudied. However, oxygen therapy, smoking cessation, inhaled bronchodilators should be prescribed for these patients as usual. Corticosteroids should be used for patients with CTD related CPFE (CTD-CPFE) and they usually have better clinical outcomes than non CTD-CPFE patients (9). The major complications of CPFE that have a profound impact of survival statistics described earlier are as follows:
- Pulmonary arterial hypertension (PH): the prevalence of PH has been reported to be more common in patients with CPFE (47% to 90%) than in patients with emphysema (10% to 30%) and advanced IPF (31% to 46%) (21,22). One-year survival rate of only 60% was reported in a study involving 40 CPFE patients with PH confirmed by right heart catheterization (23). Unfortunately, the impact of using pulmonary vasodilators has not been extensively studied and a possibility of worsening hypoxemia exists due to ventilation-perfusion mismatch resulting from such treatment (7);
- Lung cancer: patients with CPFE develop lung cancer more commonly (35.8% to 46.8%) than patients with IPF (22.4% to 31.3%) and those with emphysema alone (6.8% to 10.8%) (10,24). Interestingly, squamous cell carcinoma being the most common histologic type of cancer in these patients (24). Lung cancer in patients with CPFE and IPF predominantly occurs in the sub-pleural areas unlike those with COPD where it occurs more frequently in the lung apices (24). This distribution suggests that the emphysema by itself might not contribute as much as lung fibrosis to the development of lung cancer in CPFE;
- Acute lung injury: a very high incidence of acute lung injury after lung resection surgery or chemotherapy has been noted and up to 70% of post-lobectomy ARDS cases (70%) were noted to have preexisting CPFE (25). A 19.8% incidence of acute lung injury following definitive lung cancer therapy was reported among 101 patients with CPFE and lung cancer. Surgical resection was the most common cause of acute exacerbation (27.3%) followed by chemotherapy (20%) and radiation (16.7%) alone (26).
In view of such poor survival data associated with this syndrome and the lack of a definitive management strategy, it is reasonable to offer lung transplantation to all patients with CPFE as soon as they are diagnosed (27). Our patient underwent a rapid pre-transplant workup and has undergone a successful double-lung transplantation one week ago.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Cottin V, Nunes H, Brillet PY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J 2005;26:586-93. [Crossref] [PubMed]
- Cottin V. The impact of emphysema in pulmonary fibrosis. Eur Respir Rev 2013;22:153-7. [Crossref] [PubMed]
- Ye Q, Huang K, Ding Y, et al. Cigarette smoking contributes to idiopathic pulmonary fibrosis associated with emphysema. Chin Med J (Engl) 2014;127:469-74. [PubMed]
- Ryerson CJ, Hartman T, Elicker BM, et al. Clinical features and outcomes in combined pulmonary fibrosis and emphysema in idiopathic pulmonary fibrosis. Chest 2013;144:234-40. [Crossref] [PubMed]
- Washko GR, Hunninghake GM, Fernandez IE, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med 2011;364:897-906. [Crossref] [PubMed]
- Sakai F, Tominaga J, Kaga A, et al. Imaging diagnosis of interstitial pneumonia with emphysema (combined pulmonary fibrosis and emphysema). Pulm Med 2012;2012:816541.
- Mercurio V, Carlomagno G, Fazio S. Response to pulmonary vasodilator treatment in a former smoker with combined interstitial lung disease complicated by pulmonary hypertension: case report and review of the literature. Heart Lung 2012;41:512-7. [Crossref] [PubMed]
- Odani K, Yoriko M, Shoji Y. Computed tomographic evaluation in the cases of coexistence of pulmonary emphysema with idiopathic pulmonary fibrosis. Journal of Tomography (Japan) 2004;31:25-9.
- Cottin V, Nunes H, Mouthon L, et al. Combined pulmonary fibrosis and emphysema syndrome in connective tissue disease. Arthritis Rheum 2011;63:295-304. [Crossref] [PubMed]
- Inomata M, Ikushima S, Awano N, et al. An autopsy study of combined pulmonary fibrosis and emphysema: correlations among clinical, radiological, and pathological features. BMC Pulm Med 2014;14:104. [Crossref] [PubMed]
- Kitaguchi Y, Fujimoto K, Hanaoka M, et al. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology 2010;15:265-71. [Crossref] [PubMed]
- Mori K, Shirai T, Mikamo M, et al. Respiratory mechanics measured by forced oscillation technique in combined pulmonary fibrosis and emphysema. Respir Physiol Neurobiol 2013;185:235-40. [Crossref] [PubMed]
- Lin H, Jiang S. Combined pulmonary fibrosis and emphysema (CPFE): an entity different from emphysema or pulmonary fibrosis alone. J Thorac Dis 2015;7:767-79. [PubMed]
- Katzenstein AL, Mukhopadhyay S, Zanardi C, et al. Clinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimens. Hum Pathol 2010;41:316-25. [Crossref] [PubMed]
- Monso E, Tura JM, Marsal M, et al. Mineralogical microanalysis of idiopathic pulmonary fibrosis. Arch Environ Health 1990;45:185-8. [Crossref] [PubMed]
- Roshan R, Guptal M, Kulshrestha R, et al. Combined pulmonary fibrosis and emphysema in a welder. Monaldi Arch Chest Dis 2012;77:26-8. [PubMed]
- Karkhanis VS, Joshi JM. Combined pulmonary fibrosis and emphysema in a tyre industry worker. Lung India 2012;29:273-6. [Crossref] [PubMed]
- Cottin V, Reix P, Khouatra C, et al. Combined pulmonary fibrosis and emphysema syndrome associated with familial SFTPC mutation. Thorax 2011;66:918-9. [Crossref] [PubMed]
- Epaud R, Delestrain C, Louha M, et al. Combined pulmonary fibrosis and emphysema syndrome associated with ABCA3 mutations. Eur Respir J 2014;43:638-41. [Crossref] [PubMed]
- Nunes H, Monnet I, Kannengiesser C, et al. Is telomeropathy the explanation for combined pulmonary fibrosis and emphysema syndrome?: report of a family with TERT mutation. Am J Respir Crit Care Med 2014;189:753-4. [Crossref] [PubMed]
- Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013;62:D109-16. [Crossref] [PubMed]
- Portillo Carroz K, Roldán Sánchez J, Morera Prat J. Combined pulmonary fibrosis and emphysema. Arch Bronconeumol 2010;46:646-51. [PubMed]
- Cottin V, Le Pavec J, Prévot G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J 2010;35:105-11. [Crossref] [PubMed]
- Kwak N, Park CM, Lee J, et al. Lung cancer risk among patients with combined pulmonary fibrosis and emphysema. Respir Med 2014;108:524-30. [Crossref] [PubMed]
- Saito H, Minamiya Y, Nanjo H, et al. Pathological finding of subclinical interstitial pneumonia as a predictor of postoperative acute respiratory distress syndrome after pulmonary resection. Eur J Cardiothorac Surg 2011;39:190-4. [Crossref] [PubMed]
- Usui K, Tanai C, Tanaka Y, et al. The prevalence of pulmonary fibrosis combined with emphysema in patients with lung cancer. Respirology 2011;16:326-31. [Crossref] [PubMed]
- Dias OM, Baldi BG, Costa AN, et al. Combined pulmonary fibrosis and emphysema: an increasingly recognized condition. J Bras Pneumol 2014;40:304-12. [Crossref] [PubMed]


