Pancreatic cancer from bench to bedside: molecular pathways and treatment options
Introduction
It is known that pancreatic cancer (PC) is a lethal disease with a 5-year survival rate of 1.2–6% encompassing the pancreatic ductal adenocarcinoma that accounts for 85–95% of all pancreatic malignancies (1,2). Current data indicate that it is the seventh and fourth leading cause of cancer-related death worldwide. In the near future by 2030 it will become the second leading cause in the United States (3). During the past 30 years the incidence of pancreatic cancer has increased in developed countries, along with an increase in mortality rates. Increased mortality rates are observed in both sexes (4-7). Current statistical data indicate that there is an increased need for efficient and well-tolerated treatment options in pancreatic cancer. Below we will present current treatment options and the molecular pathways currently investigated. Also, we will provide all novel clinical trials and agents under development that may provide a rationale for future investigations.
Clinical presentation, signs, and symptoms
Unfortunately, pancreatic cancer is usually diagnosed at advanced stage. The most common early disease symptoms are: weight loss, back pain, abdominal pain, nausea and vomiting, dyspepsia, new-onset diabetes, bloating,changes in bowel habit, lethargy,pruritus, shoulder pain, and jaundice (8). In a previous published study, lethargy [OR 1.42 (1.25–1.62)], back pain [odds ratio (OR) 1.33; (95% CI 1.18–1.49)], and new-onset diabetes [OR 2.46 (2.16–2.80)] were observed features of pancreatic cancers (8). The following five symptoms were observed to occur more than 6 months before diagnosis: dysphagia, back pain, changes in bowel habit, shoulder pain, and lethargy (8). In a previous published study it was observed that lethargy, and depression as the first symptoms in about 38–45% of patients with pancreatic cancer (9). On another occasion a recent review reported nine presenting symptoms in patients with advanced pancreatic cancer (10). In this study abdominal pain and diabetes, were frequently reported in advanced pancreatic cancer (11). Usually in several studies up to 25% of the patients report upper abdominal discomfort up to six months prior to diagnosis (12).
Current management of pancreatic cancer
Surgical resection
Only 20% of patients will be diagnosed with early pancreatic cancer and for these patients, surgical resection is the treatment of choice (13,14). However; after complete resection adjuvant chemotherapy with gemcitabine or 5-fluorouracil and/or chemo-radiation has to follow and still prognosis remains disappointing (15). In the past years randomized controlled trials (16-18) have demonstrated increased overall survival (OS) with adjuvant therapy and this observation is considered the most important advancement in the treatment of pancreatic cancer (13). There are also cases were neoadjuvant chemotherapy is proposed to improve surgical margins in order for the patient to become operable (19-22) (Table 1).

Full table
Locally advanced and metastatic disease
The chemotherapy agent gemcitabine or gemcitabine-based combination chemotherapy has been vastly used and is the most acceptable first line treatment for advanced pancreatic cancer. However, still the median survival (MS) remains approximately 9 mo (13,23,24). In a recent study “FOLFIRINOX” (folinic acid, 5-fluorouracil, irinotecan, oxaliplatin) an advantage in survival and quality of life was observed when compared to gemcitabine alone. In specific; this combination significantly improved the OS, progression-free survival in pancreatic cancer patients (25). Almost the same effectives was observed with nab-paclitaxel plus gemcitabine (26). It has been observed that almost 10% of patients who have received have survived two years, which is a rare event in advanced disease (27). However, an issue that has to be taken under consideration is the fact that these regimens have increased toxicities and therefore they have to be administered to patients with good performance status (25,26). The care of patients with poor performance status or metastatic disease remains palliative, since gemcitabine based therapies have limited efficacy, however, local therapies can be considered (28).
Targeted therapy
Targeted treatment based on the genome of the tumor has revolutionized current cancer treatment. Pharmacogenetics seems to be one of the treatment options for pancreatic cancer (29). Unfortunately genetically heterogenicity has been observed in pancreatic cancer (30), however; there are several targeted therapies as treatment options, such as; small molecule inhibitors and monoclonal antibodies which inhibit constitutively-active cell surface signaling molecules. Currently results of phase I–III clinical trials presented by Seicean et al. (31) did not present favorable results due to the resistance from KRAS2 mutations and upregulation of alternate signaling pathways (13,32). Until now only the tyrosine kinase inhibitor of epidermal growth factor receptor, “erlotinib”, has been approved agent, in combination with gemcitabine, and offers increase in survival of two weeks (33) (Figure 1).
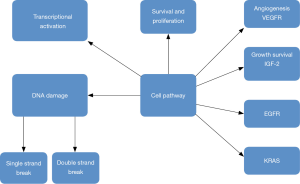
Cell metabolism in pancreatic cancer
In order for normal cells to alter their proliferation rate several metabolic pathways are reprogrammed (34). The increased metabolism of cancer cells is one the most important features of this disease that currently is being targeted as treatment (35). Cancer cells adapt their metabolic needs based on their environment. Recent studies have enlighten several metabolic routes and signaling pathways that control tumor progression (36). The most important pathways are considered the following: glutamine regulatory enzymes, Ras signaling, lipid metabolism and autophagy (37-39). Pancreatic cancer as most cancer types are characterized by a high increase in glucose uptake and metabolism (40) and high glycolytic rates the so called “Warburg effect”. Moreover; it is known that cancer cells compared to normal cells have specific metabolic dependencies, they have increased need of the amino acid glutamine (41). Kras is responsible for reprogramming glutamine metabolism in PDAC (42). Targeting the Warburg effect has shown that the p53 status of PDAC determined the response to inhibitors of the enzyme lactate dehydrogenase-A (43). Recently it has been investigated and observed that cancer cells not only fuel from glucose and glutamine but also these cells utilize amino acids as well as lipids and protein (44). It has been observed that early breakdown of proteins and subsequent increase in plasma levels of branched-chain amino acids are early events in pancreatic cancer progression as of course observed in other cancer types (45). In a recent published work it was suggested that mitochondrial respiration is another potential functional target to manage pancreatic cancer (46). In the study by Viale et al. it was revealed that tumors can relapse 2–4 months after Kras mutation withdrawal. However, it was observed that after Kras mutation extinction, they found a sub-population of surviving cells that which did not depend on Kras and anabolic glucose metabolism as the primary tumor but they had a strong reliance on mitochondrial energy production (46). Based on this observation it is suggested that in order to eliminate pancreatic tumors and prevent disease relapse we should target both populations, with inhibitors of glucose and mitochondrial metabolism. Mitochondrial metabolism regulation will be the next treatment option for pancreatic cancer. In pancreatic cancer high levels of autophagy are observed due to a transcriptional program that regulates lysosome biogenesis and nutrient scavenging (47,48). Blockage of autophagy in pancreatic cancer was achieved with genetic inhibition and drug administration both in vitro and in vivo (48,49). However, it has been observed that tumor progression in pancreatic cancer is genotype-dependent and autophagy blockage is not the best treatment option (50). Nevertheless, it is still not established whether p53 status affects PDAC response to autophagy inhibition (51). In pancreatic cancer micropinocytosis of protein and lysophospholipids as source of fatty acids has been observed as a food source. Macropinocytosis is a process where extracellular fluids through specialized vesicles named macropinosomes are inserted into the cells. It has been observed that Ras proteins regulate this process by which cancer cells internalize extracellular proteins, glutamine still remains the most valuable amino acid (52,53). Moreover; Ras-dependent mechanism of scavenging of fatty acid has been shown in pancreatic cancer cells (54). It has been observed that due to the high demand of fatty acids by cancer cells, these cells have found mechanisms of fatty acid supply, such as the uptake of extracellular lipids, in order to face cancer growth requirements. Until now there was an increasing interest in glucose metabolism and aerobic glycolysis, however; it is becoming clearly evident that the alteration of lipid metabolism is critical for cancer cell metabolism (55). Enzymes involved in lipogenesis and lipolysis have been found overexpressed in pancreatic cancer, in particular fatty acid synthase (FASN) that catalyses the final steps of fatty acid synthesis and ATP citrate lyase (39). It has been observed that elevated levels of FASN protein in cancer cells and in serum of pancreatic cancer patients are associated with poor prognosis (39). In cancer cells hyper activation of lipogenesis is observed and characterized by an increase in the degree of lipid saturation compared with non-lipogenic tumors (56). Therefore, the rise in saturated and monounsaturated lipids in cancer cell membranes increases the resistance to oxidative stress, since polyunsaturated lipids are more susceptible to lipid peroxidation. Pancreatic cancer cells use alternative routes to lipogenesis in order to obtain fatty acids, primarily through uptake of extracellular lipids derived from diet, liver synthesis or released by the adipose tissue. Moreover; current evidences suggest that pancreatic cancer is highly dependent on cholesterol (57). It has been observed that the elevated requirement of cholesterol by pancreatic cancer cells can be supplied by de novo synthesis, receptor mediated uptake of cholesterol-rich low-density-lipoproteins (LDL) by the LDL receptor (LDLR), or by hydrolysis of cholesteryl ethers (CE) that accumulate in specific lipid droplets (LDs) that are thought to act as storage of triacylglycerol and CE (57). It has been observed that cholesterol uptake, is activated in tumors (57). Interestingly an increase in the amount of cholesterol and overexpression of the low-density lipoprotein receptor (LDLR) in pancreatic tumor cells is observed (57). Based on this observation LDLR has been proposed as a novel metabolic target to limit pancreatic cancer progression. It has been previously observed in pancreatic tumor cells that shRNA silencing of LDLR reduces considerably cholesterol uptake and alters its distribution, decreases tumor cell proliferation, and limits the activation of ERK1/2 survival pathway. Moreover; with cholesterol uptake blockage the effect of chemotherapy on pancreatic cancer regression to chemotherapeutic agents is increased. It has been observed that high LDLR expression in pancreatic cancer is not associated with tumor stage, however; it has been shown to correlate to a higher risk of disease recurrence. Based on these findings it is suggested that pancreatic cancer cells are highly dependent on cholesterol uptake, and that either this process or LDLR is a promising metabolic target to use in combination with chemotherapy. Lipid metabolism in pancreatic cancer is that pancreatic cancer cells do not rely solely in de novo synthesis of lipids but also utilize circulating and diet derived lipids. Therefore it can be said that high dietary intake and obesity is a risk factor in pancreatic cancer.
Pancreatic cancer and its precursors
Pancreatic cancer is usually diagnosed at late disease and therefore it has a poor prognosis with a mortality rate almost equaling to the incidence rate (58,59). Therefore tools for early disease are in need. Moreover; deciphering the factors important for pancreatic cancer progression will help to identify novel treatment options as mentioned in the previous paragraphs. It has been observed that pancreatic cancer can develop from three established precursor lesions (60): (I) intraductal papillary mucinous neoplasm (IPMN); (II) pancreatic intraepithelial neoplasia (PanIN) and the cystic lesions; and (III) mucinous cystic neoplasm (MCN). The majority of pancreatic cancer is thought to arise from PanINs and less frequently from IPMN, whereas MCNs are rare (61). There is indirect evidence for PanINs as precursors for pancreatic cancer, which is largely based on the fact that pancreatic cancer is often associated with advanced PanIN, and both share common tumor promoting genetic alterations. On the other hand, cystic lesions can directly be identified as the origin for PDA on histological examination and imaging techniques such as MRI scan or endoscopic ultrasound (EUS). Due to more frequent and better diagnostic imaging as well as physicians’ awareness, IPMN lesions are increasingly identified in the pancreas, the ideal management of these patients is still an ongoing debate (62). It has been observed that pancreatic cancer that is associated with IPMNs has a much more favorable prognosis than pancreatic cancer that arises from PanINs (63-65). A possible explanation could be different genetic mutations during evolution of pancreatic cancer from its precursors. It has been observed that KRAS mutation occurs nearly universally during PanIN initiation (66), KRAS is less frequently mutated in IPMNs (67,68). IPMNs but not PanINs frequently harbor mutations in GNAS and RNF43 (69-71). Moreover; common genetic alterations in both precursors are found in TP53 and CDKN2A [reviewed by Xiao (72) and Gnoni et al. (61)]. Furthermore; the different biology of PanIN and IPMN-associated with pancreatic cancer could be the different cellular origin of the precursors. Recent evidence from genetically engineered mouse models (GEMM) revealed that the cellular origin of PanINs and IPMNs might be different (73-75).
Targeted therapy in PDAC
Epidermal growth factor receptor (EGFR) pathway inhibitors
Epidermal growth factor receptor (EGFR) is a transmembrane receptor member of the ErbB family with a tyrosine kinase domain that is activated by many ligands including epidermal growth factor (EGF), amphiregulin, epiregulin, tumor growth factor-α (TGF-α), heparin-binding EGF, betacellulin and neuregulin. EGFR is involved in cell cycle regulation, adhesion, differentiation and cell survival, through activation of the Ras/MAP kinase, Janus kinase/Stat, phospholipase C/protein kinase C and phosphatidylinositol 3’-kinase (PI3K)/Akt pathways. In previous studies it was observed that EGFR is overexpressed in up to 90% of pancreatic cancer samples. Based on this findings tyrosine kinase inhibitorstargeting EGFR have been considered a promising therapeutic agent (76). Eroltinib is a tyrosine kinase inhibitor (TKI) molecule that competes with ATP for binding to the kinase domain, thereby blocking downstream signal transduction of EGFR. A large phase III trial, enrolling 569 chemotherapy naïve patients with locally advanced or metastatic pancreatic adenocarcinoma randomized to receive gemcitabine plus placebo or gemcitabine plus erlotinib 100–150 mg daily was performed. The median overall survival (mOS) and progression free survival (PFS) were modestly, but statistically significantly, improved in the combination arm, 6.24 vs. 5.91 mo (P=0.038) and 3.75 vs. 3.55 mo (P=0.004), respectively (33). Both EGFR status nor KRAS status analysed in the subgroup of patients treated with erlotinib was associated with survival benefit in patients receiving the combination schedule (77).
For now erlotinib has been approved by the FDA in combination with gemcitabine as a first-line treatment for advanced pancreatic adenocarcinoma. Cetuximab is a monoclonal antibody binding the extracellular domain of epidermal growth factor receptor. Unfortunately the encouraging results in a phase I trial, were not observed again in subsequent studies (gemcitabine-based chemotherapy) failed to demonstrate any survival benefit (78,79). Gefitinib; another tyrosine kinase inhibitor of ATP binding to the intracellular kinase domain of EGFR, in combination with gemcitabine in inoperable or metastatic pancreatic cancer patients was administered in a phase II trial. The combination demonstrated promising activity with a mOS and PFS in the combination arm of 7.3 and 4.1 mo, respectively, however; other evidence supporting a role of gefitinib in pancreatic adenocarcinoma treatment is lacking (80). HER-2, which is another ErbB family of transmembrane tyrosine kinase receptors which has been observed overexpressed in 11% of pancreatic adenocarcinoma cases. It has been observed that HER2-positive status is correlated with shorter survival (81). Trastuzumab plus gemcitabine was tested in 34 metastatic pancreatic cancer patients with HER-2 overexpression as determined by immunohistochemistry, and partial responses were observed in 6% of cases (82). Harder et al. (83) in a multicentre phase II study, investigated the efficacy and toxicity of the HER2 antibody, trastuzumab, plus capecitabine in patients with pancreatic cancer and HER2 overexpression, however; this study did not present favourable results to either PFS or OS compared with standard chemotherapy. Lapatinib another HER-2 inhibitor was approved by the FDA and clinical trials have been initiated to test the effect of this inhibitor combined with chemotherapy in pancreatic carcinoma. Lapatinib was tested in combination with capecitabine as a second-line treatment in advanced pancreatic cancer with promising initial results. However; more studies are needed to evaluate the real effectiveness and role of this molecule in the treatment of pancreatic adenocarcinoma (84). Nimotuzumab, another anti-EGFR monoclonal antibody, showed promising results (85). In the study by Strumberg et al. (86) a phase II trial showed PFS after 1 year of 10.3% and median overall survival of 18.1 wk with a tolerable toxicity profile. Currently afatinib; another TKI of EGFR, HER2 and HER4, is under evaluation in an ongoing phase II trial (87,88).
The KRAS pathway and downstream signalling cascade inhibitors
Current information regarding KRAS mutations indicated that KRAS activating mutations are present in 70% to 90% of pancreatic cancer. K-Ras is a GTPase protein belonging to the Ras protein family. This protein has oncogenic activity, and promotes activation of proliferation and inhibits apoptosis through the RAF/MEK/ERK and PIK3/AKT pathways. It is known that K-Ras pathway is very difficult to target, and currently there are no inhibitors that can actually block this pathway in clinical practice (89). In a preclinical study it was observed that that farnesylation is an important post-translational modification required for Ras activation, allowing the protein to be attached to the plasma membrane for signal transduction (90). Although farnesyl-transferase inhibitors, particularly tipifarnib, presented anti-proliferative activity in pancreatic tumour cell lines, it failed to improve overall survival either as a single agent or in combination with gemcitabine in a phase III trial (91,92). Therefore it is suggested for now to block targets downstream of KRAS, such as the protein kinase MEK. Therefore selumetinib an oral small molecule that inhibits MEK1/2 was produced. This agent was administered in a phase II trial, where patients were randomized to receive single-agent capecitabine or selumetinib as a second-line treatment for advanced pancreatic cancer. The selumetinib arm showed a median overall survival of 5.4 vs. 5.0 mo in the capecitabine arm, but this result was not statistically significant (93). Trametinib, another MEK1/2 inhibitor, has been tested in pancreatic cancer in combination with gemcitabine against a regimen of gemcitabine plus placebo in a phase II randomized multicentre study. However; again no significant advantages were demonstrated in terms of overall survival or PFS (94). Rigosertib, a first-in-class Ras mimetic and small molecule inhibitor of multiple signalling pathways, including polo-like kinase 1 and phosphoinositide 3-kinase (PI3K), was administered in combination with gemcitabine in patients with treatment-naïve metastatic pancreatic adenocarcinoma in a phase II/III randomized study. In the combination regimen no improved of survival or response was observed, as recently presented at the 2015 ASCO Annual Meeting (95). Research in this field is continuing.
IGFR pathway inhibitors
Insulin like growth factor 1 receptor (IGFR-1) is another possible target for pancreatic cancer, which is highly expressed in pancreatic cells. It has been observed that upon ligand binding, it activates several pathways involved in cell proliferation and cell survival such as the PIK3/AKT pathway (96). Moreover, other agents such as; monoclonal antibodies against IGFR (cixutumumab, ganitumab) were evaluated in pancreatic cancer treatment, however; they failed to show a statically significant survival benefit (97). In a phase III trial assessing ganitumab in combination with gemcitabine, the study closed early based on a pre-planned futility analysis. The median overall survival was 7.1 mo in the maximum dose ganitumab arm vs. 7.2 mo in the placebo arm (HR 0.97, P=0.397) (98).
Angiogenesis pathway inhibitors
Neo-angiogenesis is known to be an essential metastatization and tumor progression mechanism. Vascular endothelial growth factor (VEGF) is known to stimulate proliferation of endothelial cells and it has been observed to be overexpressed in human pancreatic cancer. However; current data indicate that neo-angiogenesis inhibitors, particularly VEGF inhibitors, have not presented favorable overall survival in combination with gemcitabine in advanced pancreatic cancer. In two phase III trials that tested the efficacy of bevacizumab in association with gemcitabine alone, or gemcitabine plus erlotinib, did not confirm previous findings (99,100). A new recombinant fusion protein, aflibercept which has extracellular portions of VEGFR-1 and VEGFR-2, which binds VEGF-A, VEGF-B and placental growth factors 1 and 2 thereby inhibiting VEGF-ligand-dependent signaling (101) processes, was observed to suppress tumor growth in pancreatic cell lines and xenografts. However; in a phase III study adding aflibercept to gemcitabine did not improve OS in metastatic pancreatic cancer patients (102). Similarly sorafenib, an oral multikinase inhibitor of Raf-kinase, VEGF-R2/-R3 and PDGFR-β, and axitinib, an anti-angiogenesis agent did not present any statistically significant efficacy in advanced pancreatic adenocarcinoma (103,104). Currently there are phase II studies combining chemotherapy with promising new anti-angiogenic molecular agents, such as TL-118, which is a nonsteroidal anti-inflammatory oral medication, or necuparanib, which is re-engineered from heparin with possible anti-tumor activity. The results of these studies will be published soon (105,106).
Embryonic pathway inhibitors
The Hedgehog signaling is known to have a critical role in cell proliferation and survival during the embryonic development. In normal pancreatic cells this pathway is silenced, however; pathological activation is observed in many solid tumors. In pancreatic cancer this signaling pathway is found overexpressed. Hedgehog binds to the extracellular receptor Patched, which, in the absence of Hedgehog, suppresses activation of the G-protein-coupled receptor Smoothened and upregulates glioma associated oncogene homolog1 transcriptional activity (107). In the study by Bailey et al. (108) it was observed that Sonic hedgehog (SHH) was detected in precursor lesions and in pancreatic cancer tumor samples which contributed to the formation of the desmoplastic reaction. This characteristic of pancreatic cancer limits the effective delivery of anticancer agents to pancreatic cancer cells. Genetically engineered mouse models demonstrated a depletion of tumor matrix from Sonic hedgehog SHH pathway inhibition this treatment option could be the strategy in pancreatic cancer therapy (109). Currently, vismodegib (GDC-0449), an oral small-molecule inhibitor targeting Smoothened (110), is administered in an open phase II trials in combination with gemcitabine in advanced cancer, in combination with gemcitabine and nab-paclitaxel in metastatic settings. Current results provide favorable outcome (111), and as a single agent in neoadjuvant settings followed by surgery (112-114). The Smoothened inhibitor saridegib (IPI-926) was administered in combination with gemcitabine against gemcitabine plus placebo in a randomized, double-blind, placebo-controlled phase II trial. In this trial patients with metastatic disease were enrolled. The study was terminated due to early decreased of patient survival in the saridegib arm (115). Hedgehog inhibitors are currently an active research field, and the results of several clinical trials are under way (116). Notch signaling is another embryonic pathway crucial for pancreatic organogenesis, this pathway is silenced after pancreas development, however; it is active only in a stem cell subgroup. This pathway has been observed to be upregulated pancreatic cancer and promotes tumourigenesis. Binding of Notch ligand to its receptor promotes a cascade of proteolytic cleavages, mediated by γ-secretase. The activated form ICN (intra cellular notch) forms part of a transcription complex that, after translocating to the nucleus, regulates transcription of several genes involved in proliferation and differentiation of cells. It has been observed that interacts with other pathways such as Hedgehog, KRAS and NF-κB signaling (117,118). The selective inhibitor RO4929097 of the γ-secretase enzyme has anti-tumor activity in preclinical studies (119). In a recent phase II single-arm trial RO4929097 was assessed, enrolling 18 previously treated advanced pancreatic cancer patients. The treatment was well tolerated; the median survival was 4.1 mo, and the median progression-free survival was 1.5 mo (120). Encouraging clinical results were observed testing demcizumab, an anti-Delta-like ligand 4 antibody, plus gemcitabine and nab-paclitaxel in advanced PDAC in a phase b trial. However; more studies are in need to confirm these preliminary data (121).
Poly ADP-ribose polymerase (PARP) inhibitors
Mutations that affect breast cancer pathway components, and especially the tumor suppressor gene breast cancer-2 (BRCA2) gene, which is associated with hereditary predisposition to breast, ovarian and pancreatic cancer are known to promote deficiency in DNA damage repair mechanisms and also induce genome instability (122). Poly ADP-ribose polymerase (PARP) is a nuclear enzyme recruited to repair cell DNA damage, patients with defects in the homologous DNA recombination pathway may benefit from the use of PARP inhibitors. This treatment can be applied as monotherapy or in combination with radiation or other chemotherapeutic agents. Clinical trials testing poly ADP ribose polymerase (PARP) inhibitors are currently under development phase (123-125).
mTOR and PI3K/Akt pathway inhibitors
It is known that after activation, Ras can phosphorylate PI3K, which in turn activates Akt, a serine/threonine kinase. Signal transduction by activated PI3K/Akt plays a role in tumor cell proliferation, metabolism and survival. This occurs through several downstream targets, such as; the mammalian target of rapamycin (mTOR) (126). Currently there are trials testing PI3K/AKT axis inhibitors in advanced pancreatic cancer patients after encouraging preclinical model results (127). There are several PI3K/AKT axis inhibitors being tested such as: RX-0201, an Akt antisense oligonucleotide tested in a phase II study plus gemcitabine; BKM120, a PI3K inhibitor tested in combination with the mFOLFOX-6 schedule; and BEZ235, a combined inhibitor of PI3K and mTOR assessed in a phase study in combination with the MEK inhibitor MEK162 (128-130). In the study by Wolpin et al. (131) everolimus, an oral mTOR inhibitor, was administered as monotherapy in 33 gemcitabine-refractory pancreatic cancer patients. The PFS and OS observed were 1.8 and 4.5 mo, respectively. In a recent published phase II study (single arm) where everolimus was tested in combination with capecitabine the median OS was 8.9 mo and PFS was 3.6 mo (132). In the recent future we are anticipating the results of a phase I/II study testing everolimus in combination with gemcitabine in advanced settings and the results of a phase II trial testing temsirolimus (mTOR inhibitor) (133,134).
Tumor stroma inhibitors
In pancreatic cancers a dynamic compartment (stroma) has been observed to be critically involved in tumor formation, progression and metastatic process. Stroma microenvironment and consisting elements are considered a treatment target in addition to previously described trials evaluating Hedgehog signaling inhibitors (135). Currently a phase II trial is recruiting patients I order to administer PEGPH20, a pegylated formulation of recombinant hyaluronidase. The study design is to enrol untreated patients with metastatic disease in order to receive a combination of PEGPH20, nab-naclitaxel and gemcitabine or a combination of nabpaclitaxel and gemcitabine (136,137). Moreover; it has been observed that that inhibition of platelet derived growth factor receptor (PDGFR), could be a possible treatment target. This receptor is expressed in stromal cells and has a critical role in recruiting pericytes and in interstitial fluid pressure in the tumor stroma. The importance of this pathway was suggested by preclinical studies using an orthotopic pancreatic tumor mouse model (138). TKI258, a PDGFR inhibitor, is currently under evaluation in a phase I dose assessment for advanced pancreatic cancer patients (139). Moreover; matrix metalloproteinases (MMPs) are a family of proteolytic enzymes responsible for the degradation of connective tissue proteins. Matrix metalloproteinase proteins aberrant expression is observed in pancreatic adenocarcinoma. Marimastat has been previously tested. However; the results of a phase III trial which used marimastat with gemcitabine in patients with advanced pancreatic cancer did not present favorable results (140).
Oncolytic virotherapy
It has been suggested that viruses could be a strategic tool for targeting tumors. This is due to the observation that viruses activate similar pathways as tumors, and viral infections activate both the innate and adaptive immune responses (75). It has been observed that tumors create a niche of innate and adaptive immune suppression, which not only protect the tumor from the host immune system. Additionally, it limits its ability to respond to viral infection (141). In early case reports tumor regression was observed following a naturally occurring viral infection (142). The propose strategy suggests that tumor-targeted oncolytic viruses (TOVs) could selectively infect, replicate in, and lyse tumor cells, sparing healthy, normal tissues. There are two approaches for tumor-targeted oncolytic viruses the inherently tumor-selective, i.e., are naturally nonpathogenic to humans and sensitive to antiviral signaling (143) or secondly to depend on oncogenic signaling pathways such as constitutively-activated Ras (144,145). TOVs can also be genetically engineered to be tumor-selective. In order to achieve this deletions of genes are required for replication in normal tissues.(144) Deletion could involve deletion of thymidine kinase UL23 gene in herpes simplex virus (HSV)-1 (146) or thymidine kinase and vaccinia growth factor (VGF) for vaccinia virus (VV) (147). In recently published literature, gene silencing by RNA interference technology to achieve tumor selectivity has also been utilized (145). Furthermore; another strategy used is to involve the insertion of a tumor-specific promoter (148,149), by doing this the expression of a gene necessary for viral replication in order to restrict its replication in tumor cells is overexpressed (145). TOVs can also be engineered to express cell surface receptors unique to tumor cells (150,151), which allow specific tropism (145). TOVs can designed to express immunomodulatory transgenes (144). In contrast to the pharmacokinetics of the usual drug administration, the therapeutic dose of TOVs increases with time as the virus replicates and spreads to neighboring cells (144). Again as every virus particle carries a therapeutic gene, each viral progeny will also carry the transgene, in this mode it enhances the therapeutic effect (152). TOVs have the ability to directly lyse infected malignant cells and cause acute tumor debulking. However; another major advantage, is the ability of the virus to spread and potentiate an inflammatory response that allows the destruction of a tumor. This feature distinguishes TOVs from vaccines or immune adjuvants (153). Moreover; another unique feature of TOVs is to target silmutaneously multiple cellular pathways and therefore the risk of tumor resistance development is low (144). Another observation suggested that TOVs act synergistically with conventional chemotherapy and radiation (154-156). The first TOV to undergo a clinical trial was ONYX-015 (dl1520), an adenovirus deficient in the E1B gene (157). The gene product E1B-55 kDa protein was originally thought to sequester p53, inactivating it and as a result, allowing replication in a cell (158). In the study by O’Shea et al. (159) later determined that it was for the differential viral RNA export between normal and cancer cells which accounts for ONYX-015’s tumor-selectivity. Until now the first approved oncolytic virus to date is H101 (Oncorine; Shanghair Sunway Biotech, Shanghai, China), which was approved for combination with chemotherapy in China in 2005 for the treatment of head and neck cancers (157).
Discussion
The exploration of the genome of pancreatic cancer is currently the best therapeutic strategy as it has important clinical relevance. However; current efforts to understand the tumor genome profile and identify efficient targeted therapies have not presented favorable results. Several targeted agents which have been mentioned in the previous sections, almost all have failed to demonstrate efficacy in late phase clinical trials. Until now only the tyrosine kinase inhibitor erlotinib has been approved by the FDA for advanced pancreatic cancer treatment, however; the improvement of overall survival was barely 2 weeks compared with gemcitabine alone (33). Pancreatic cancer has extreme genomic heterogeneity and this is the most important reason which blocks the identification of new actionable molecular targets or to testing existing biological therapies which have already been approved for human use for other cancers. Since targeted agent administration as a single agent has not presented favorable results, multitargeted agents multitargeted agents or molecular agent combinations are in the development phase in order to inhibit more than one pathway simultaneously and to prevent or evade resistance. The majority of trials until now have combined target agents with gemcitabine, but actually, the first-line schedules are represented by FOLFIRINOX or gemcitabine plus Nabpaclitaxel. It is suggested that greater efficacy may be obtained from the combination of target agents with those chemotherapeutic drugs. Furthermore, in most studies in which molecular or chemotherapeutic agents in pancreatic cancer were tested, patients from unselected population were enrolled to treat. In the past three years, 116 trials specific for pancreatic cancer systemic therapy were registered of which only about 8% applied criteria to select a patient subset based upon molecular biomarkers (160). In order to stratify patients, the Australian Pancreatic Cancer Genome Initiative has started a pilot study to evaluate the feasibility of assessing a more stratified approach in the management of pancreatic cancer through predefined actionable molecular phenotypes. Patients are enrolled in this trial, called IMPaCT (Individualised Molecular Pancreatic Cancer Therapy). Firstly, a preliminary phenotype screening in performed in order to compare the use of gemcitabine in an unselected population to a stratified approach. The major target of the study is to create a tailored approach to pancreatic cancer treatment, as this seems to be the major challenge for the future of pancreatic cancer treatment (161,162). Moreover; based on the advancements of biotechnology, biological agents can find application in cancer treatment by tumor-targeted delivery of cytotoxic drugs. In the study by Ahn et al. (162) a developed antibody fragment was installed in polymeric micelles via maleimide-thiol conjugation for selective delivery of platinum drugs to pancreatic tumors. It was observed that this antibody-drug conjugate significantly suppressed the growth of pancreatic tumor xenografts. This technology advancement, which has activity in vitro and in a mouse model, could be a promising future strategy in pancreatic cancer therapy not only as a systematic therapy, but also as a local treatment (28,162). In conclusion, the lack of efficacy of targeted therapy in PDAC represents a challenge for the future, and more efforts are needed in order to make pancreatic cancer a curable disease. Future steps include the development of novel conjugates for efficient drug delivery, further exploration of the pancreatic genome and extensive evaluate of combination therapeutic strategies with non-specific cyto-toxic agents and targeted agents in order to maximize clinical benefit (7). The ability of TOVs to stimulate inflammation, deliver genes and immunomodulatory agents as well as reduce tumor burden by direct cell lysis, could be a novel therapeutic approach, however; more trials are in need. Probably this treatment approach could be used simultaneously with non-specific cyto-toxic agents or as an adjuvant treatment. Again local therapies could be considered to reduce tumor burden.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Bosetti C, Bertuccio P, Malvezzi M, et al. Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980. Ann Oncol 2013;24:2657-71. [Crossref] [PubMed]
- Malvezzi M, Bertuccio P, Levi F, et al. European cancer mortality predictions for the year 2014. Ann Oncol 2014;25:1650-6. [Crossref] [PubMed]
- Malvezzi M, Bertuccio P, Rosso T, et al. European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women? Ann Oncol 2015;26:779-86. [Crossref] [PubMed]
- Sharma P, Wagner K, Wolchok JD, et al. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 2011;11:805-12. [Crossref] [PubMed]
- Keane MG, Horsfall L, Rait G, et al. A case-control study comparing the incidence of early symptoms in pancreatic and biliary tract cancer. BMJ Open 2014;4:e005720. [Crossref] [PubMed]
- Cosci F, Fava GA, Sonino N. Mood and anxiety disorders as early manifestations of medical illness: a systematic review. Psychother Psychosom 2015;84:22-9. [Crossref] [PubMed]
- Sharma C, Eltawil KM, Renfrew PD, et al. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990-2010. World J Gastroenterol 2011;17:867-97. [Crossref] [PubMed]
- Li J, Li Y, Cao G, et al. Early manifestations of pancreatic cancer: the effect of cancer-nerve interaction. Med Hypotheses 2013;81:180-2. [Crossref] [PubMed]
- Grahm AL, Andren-Sandberg A. Prospective evaluation of pain in exocrine pancreatic cancer. Digestion 1997;58:542-9. [Crossref] [PubMed]
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362:1605-17. [Crossref] [PubMed]
- Shaib Y, Davila J, Naumann C, El-Serag H. The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. Population-based study. Am J Gastroenterol 2007;102:1377-82. [Crossref] [PubMed]
- Rossi ML, Rehman AA, Gondi CS. Therapeutic options for the management of pancreatic cancer. World J Gastroenterol 2014;20:11142-59. [Crossref] [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [Crossref] [PubMed]
- Lemmens VE, Bosscha K, van der Schelling G, et al. Improving outcome for patients with pancreatic cancer through centralization. Br J Surg 2011;98:1455-62. [Crossref] [PubMed]
- Takahashi H, Akita H, Gotoh K, et al. Preoperative gemcitabine-based chemoradiation therapy for pancreatic ductal adenocarcinoma of the body and tail: impact of splenic vessels involvement on operative outcome and pattern of recurrence. Surgery 2015;157:484-95. [Crossref] [PubMed]
- Nanda RH, El-Rayes B, Maithel SK, et al. Neoadjuvant modified FOLFIRINOX and chemoradiation therapy for locally advanced pancreatic cancer improves resectability. J Surg Oncol 2015;111:1028-34. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [PubMed]
- Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 2008;8:82. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:2140-1. [Crossref] [PubMed]
- Zarogoulidis P, Pavlioglou P, Pivert PL, et al. Current and future intratumoral targeted treatment for pancreatic cancer. Therapeutic delivery. 2014;5:913-26. [Crossref] [PubMed]
- Gerber DE. Targeted therapies: a new generation of cancer treatments. Am Fam Physician 2008;77:311-9. [PubMed]
- Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008;321:1801-6. [Crossref] [PubMed]
- Seicean A, Petrusel L, Seicean R. New targeted therapies in pancreatic cancer. World J Gastroenterol 2015;21:6127-45. [Crossref] [PubMed]
- Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med 2013;19:1389-400. [Crossref] [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [Crossref] [PubMed]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011;11:85-95. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Kimmelman AC. Metabolic Dependencies in RAS-Driven Cancers. Clin Cancer Res 2015;21:1828-34. [Crossref] [PubMed]
- Bryant KL, Mancias JD, Kimmelman AC, et al. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci 2014;39:91-100. [Crossref] [PubMed]
- Daye D, Wellen KE. Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Semin Cell Dev Biol 2012;23:362-9. [Crossref] [PubMed]
- Swierczynski J, Hebanowska A, Sledzinski T. Role of abnormal lipid metabolism in development, progression, diagnosis and therapy of pancreatic cancer. World J Gastroenterol 2014;20:2279-303. [Crossref] [PubMed]
- Ying H, Kimmelman AC, Lyssiotis CA, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012;149:656-70. [Crossref] [PubMed]
- Son J, Lyssiotis CA, Ying H, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013;496:101-5. [Crossref] [PubMed]
- Cohen R, Neuzillet C, Tijeras-Raballand A, et al. Targeting cancer cell metabolism in pancreatic adenocarcinoma. Oncotarget 2015;6:16832-47. [Crossref] [PubMed]
- Rajeshkumar NV, Dutta P, Yabuuchi S, et al. Therapeutic Targeting of the Warburg Effect in Pancreatic Cancer Relies on an Absence of p53 Function. Cancer Res 2015;75:3355-64. [Crossref] [PubMed]
- Mayers JR, Vander Heiden MG. Famine versus feast: understanding the metabolism of tumors in vivo. Trends Biochem Sci 2015;40:130-40. [Crossref] [PubMed]
- Mayers JR, Wu C, Clish CB, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med 2014;20:1193-8. [Crossref] [PubMed]
- Viale A, Pettazzoni P, Lyssiotis CA, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 2014;514:628-32. [Crossref] [PubMed]
- Yang S, Wang X, Contino G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev 2011;25:717-29. [Crossref] [PubMed]
- Perera RM, Stoykova S, Nicolay BN, et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature 2015;524:361-5. [Crossref] [PubMed]
- Wolpin BM, Rubinson DA, Wang X, et al. Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinoma. Oncologist 2014;19:637-8. [Crossref] [PubMed]
- Rosenfeldt MT, O'Prey J, Morton JP, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature 2013;504:296-300. [Crossref] [PubMed]
- Yang A, Kimmelman AC. Inhibition of autophagy attenuates pancreatic cancer growth independent of TP53/TRP53 status. Autophagy 2014;10:1683-4. [Crossref] [PubMed]
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013;497:633-7. [Crossref] [PubMed]
- Kamphorst JJ, Nofal M, Commisso C, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res 2015;75:544-53. [Crossref] [PubMed]
- Kamphorst JJ, Cross JR, Fan J, et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A 2013;110:8882-7. [Crossref] [PubMed]
- Baenke F, Peck B, Miess H, et al. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Disease models & mechanisms. 2013;6:1353-63. [Crossref] [PubMed]
- Rysman E, Brusselmans K, Scheys K, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res 2010;70:8117-26. [Crossref] [PubMed]
- Guillaumond F, Bidaut G, Ouaissi M, et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci U S A 2015;112:2473-8. [Crossref] [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. [Crossref] [PubMed]
- Maitra A, Fukushima N, Takaori K, et al. Precursors to invasive pancreatic cancer. Adv Anat Pathol 2005;12:81-91. [Crossref] [PubMed]
- Gnoni A, Licchetta A, Scarpa A, et al. Carcinogenesis of pancreatic adenocarcinoma: precursor lesions. Int J Mol Sci 2013;14:19731-62. [Crossref] [PubMed]
- Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183-97. [Crossref] [PubMed]
- Matthaei H, Norris AL, Tsiatis AC, et al. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2012;255:326-33. [Crossref] [PubMed]
- Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg 2010;251:470-6. [Crossref] [PubMed]
- Mino-Kenudson M, Fernandez-del Castillo C, Baba Y, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut 2011;60:1712-20. [Crossref] [PubMed]
- Kanda M, Matthaei H, Wu J, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012;142:730-3 e9.
- Amato E, Molin MD, Mafficini A, et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol 2014;233:217-27. [Crossref] [PubMed]
- Schönleben F, Qiu W, Bruckman KC, et al. BRAF and KRAS gene mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/IPMC) of the pancreas. Cancer Lett 2007;249:242-8. [Crossref] [PubMed]
- Furukawa T, Kuboki Y, Tanji E, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 2011;1:161. [Crossref] [PubMed]
- Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 2011;108:21188-93. [Crossref] [PubMed]
- Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011;3:92ra66. [Crossref] [PubMed]
- Xiao SY. Intraductal papillary mucinous neoplasm of the pancreas: an update. Scientifica (Cairo) 2012;2012:893632.
- von Figura G, Fukuda A, Roy N, et al. The chromatin regulator Brg1 suppresses formation of intraductal papillary mucinous neoplasm and pancreatic ductal adenocarcinoma. Nat Cell Biol 2014;16:255-67. [Crossref] [PubMed]
- Kopp JL, von Figura G, Mayes E, et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 2012;22:737-50. [Crossref] [PubMed]
- Melcher A, Parato K, Rooney CM, et al. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther 2011;19:1008-16. [Crossref] [PubMed]
- Tobita K, Kijima H, Dowaki S, et al. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. International journal of molecular medicine. 2003;11:305-9. [PubMed]
- da Cunha Santos G, Dhani N, Tu D, et al. Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer 2010;116:5599-607. [Crossref] [PubMed]
- Cascinu S, Berardi R, Labianca R, et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: a randomised, multicentre, phase II trial. Lancet Oncol 2008;9:39-44. [Crossref] [PubMed]
- Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 2010;28:3605-10. [Crossref] [PubMed]
- Fountzilas G, Bobos M, Kalogera-Fountzila A, et al. Gemcitabine combined with gefitinib in patients with inoperable or metastatic pancreatic cancer: a phase II Study of the Hellenic Cooperative Oncology Group with biomarker evaluation. Cancer Invest 2008;26:784-93. [Crossref] [PubMed]
- Kimura K, Sawada T, Komatsu M, et al. Antitumor effect of trastuzumab for pancreatic cancer with high HER-2 expression and enhancement of effect by combined therapy with gemcitabine. Clin Cancer Res 2006;12:4925-32. [Crossref] [PubMed]
- Safran H, Iannitti D, Ramanathan R, et al. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest 2004;22:706-12. [Crossref] [PubMed]
- Harder J, Ihorst G, Heinemann V, et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer 2012;106:1033-8. [Crossref] [PubMed]
- Safran H, Miner T, Resnick M, et al. Lapatinib/gemcitabine and lapatinib/gemcitabine/oxaliplatin: a phase I study for advanced pancreaticobiliary cancer. Am J Clin Oncol 2008;31:140-4. [Crossref] [PubMed]
- Su D, Jiao SC, Wang LJ, et al. Efficacy of nimotuzumab plus gemcitabine usage as first-line treatment in patients with advanced pancreatic cancer. Tumour Biol 2014;35:2313-8. [Crossref] [PubMed]
- Strumberg D, Schultheis B, Scheulen ME, et al. Phase II study of nimotuzumab, a humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody, in patients with locally advanced or metastatic pancreatic cancer. Invest New Drugs 2012;30:1138-43. [Crossref] [PubMed]
- Ioannou N, Dalgleish AG, Seddon AM, et al. Anti-tumour activity of afatinib, an irreversible ErbB family blocker, in human pancreatic tumour cells. Br J Cancer 2011;105:1554-62. [Crossref] [PubMed]
- PD Dr. med. Volker Heinemann. Afatinib as Cancer Therapy for Exocrine Pancreatic Tumours (ACCEPT). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT01728818, NLM Identifier: NCT01728818.
- di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology 2013;144:1220-9. [Crossref] [PubMed]
- Takashima A, Faller DV. Targeting the RAS oncogene. Expert Opin Ther Targets 2013;17:507-31. [Crossref] [PubMed]
- Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer 2011;11:761-74. [Crossref] [PubMed]
- Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 2004;22:1430-8. [Crossref] [PubMed]
- Bodoky G, Timcheva C, Spigel DR, et al. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs 2012;30:1216-23. [Crossref] [PubMed]
- Infante JR, Somer BG, Park JO, et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer 2014;50:2072-81. [Crossref] [PubMed]
- Scott AJ, O'Neil BH, Gomes C, et al. A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. J Clin Oncol 2015;33:abstr 342.
- Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther 2007;6:1-12. [Crossref] [PubMed]
- Philip PA, Goldman B, Ramanathan RK, et al. Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727). Cancer 2014;120:2980-5. [Crossref] [PubMed]
- Fuchs CS, Azevedo S, Okusaka T, et al. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol 2015;26:921-7. [Crossref] [PubMed]
- Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010;28:3617-22. [Crossref] [PubMed]
- Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 2009;27:2231-7. [Crossref] [PubMed]
- Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol 2011;12:256-62. [Crossref] [PubMed]
- Rougier P, Riess H, Manges R, et al. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer 2013;49:2633-42. [Crossref] [PubMed]
- Chiorean EG, Schneider BP, Akisik FM, et al. Phase 1 pharmacogenetic and pharmacodynamic study of sorafenib with concurrent radiation therapy and gemcitabine in locally advanced unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2014;89:284-91. [Crossref] [PubMed]
- Kindler HL, Wroblewski K, Wallace JA, et al. Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest New Drugs 2012;30:382-6. [Crossref] [PubMed]
- Ltd. TP. Investigator’s Initiated Phase II Study for Pancreatic Cancer Patients. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT01659502, NLM Identifier: NCT01659502.
- Momenta Pharmaceuticals I. M402 in Combination With Nab-Paclitaxel and Gemcitabine in Pancreatic Cancer In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT01621243, NLM Identifier: NCT01621243.
- Di Marco M, Macchini M, Vecchiarelli S, et al. Hedgehog signaling: from the cuirass to the heart of pancreatic cancer. Pancreatology 2012;12:388-93. [Crossref] [PubMed]
- Bailey JM, Swanson BJ, Hamada T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res 2008;14:5995-6004. [Crossref] [PubMed]
- Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457-61. [Crossref] [PubMed]
- Singh BN, Fu J, Srivastava RK, et al. Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms. PloS One 2011;6:e27306. [Crossref] [PubMed]
- Catenacci DV, Junttila MR, Karrison T, et al. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J Clin Oncol 2015;33:4284-92. [Crossref] [PubMed]
- National Cancer Institute (NCI). Gemcitabine Hydrochloride With or Without Vismodegib in Treating Patients With Recurrent or Metastatic Pancreatic Cancer In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT01064622, NLM Identifier: NCT01064622.
- Center. SKCC. Pancreas, Hedgehog Inhibitors for Metastatic Adenocarcinoma of the Pancreas.
- Bax L. Hedgehog Inhibition for Pancreatic Ductal Adenocarcinoma (PDAC) in the Preoperative Setting (HIPPoS). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT01096732, NLM Identifier: NCT01096732.
- Infinity Reports Update from Phase 2 Study of Saridegib Plus Gemcitabine in Patients with Metastatic Pancreatic Cancer.
- Sidney Kimmel Comprehensive Cancer Center. Gemcitabine + Nab-paclitaxel With LDE-225 (Hedgehog Inhibitor) as Neoadjuvant Therapy for Pancreatic Adenocarcinoma. In: ClinicalTrials. gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT01431794, NLM Identifier: NCT01431794.
- Sjolund J, Manetopoulos C, Stockhausen MT, et al. The Notch pathway in cancer: differentiation gone awry. Eur J Cancer 2005;41:2620-9. [Crossref] [PubMed]
- Ristorcelli E, Lombardo D. Targeting Notch signaling in pancreatic cancer. Expert Opin Ther Targets 2010;14:541-52. [Crossref] [PubMed]
- Plentz R, Park JS, Rhim AD, et al. Inhibition of gamma-secretase activity inhibits tumor progression in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 2009;136:1741-9 e6.
- De Jesus-Acosta A, Laheru D, Maitra A, et al. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drugs 2014;32:739-45. [Crossref] [PubMed]
- Hidalgo M, Cooray P, Jameson MB, et al. A phase Ib study of the anti-cancer stem cell agent demcizumab (DEM) & gemcitabine (GEM) +/- paclitaxel protein bound particles (nab-paclitaxel) in pts with pancreatic cancer. J Clin Oncol 2015;33:abstr 4118.
- Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495-501. [Crossref] [PubMed]
- Tangutoori S, Baldwin P, Sridhar S. PARP inhibitors: A new era of targeted therapy. Maturitas 2015;81:5-9. [Crossref] [PubMed]
- AstraZeneca. Study to Assess the Safety & Tolerability of a PARP Inhibitor in Combination With Gemcitabine in Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT00515866, NLM Identifier: NCT00515866.
- National Cancer Institute (NCI). Gemcitabine Hydrochloride and Cisplatin With or Without Veliparib or Veliparib Alone in Patients With Locally Advanced or Metastatic Pancreatic Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01585805, NLM Identifier: NCT01585805.
- Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 2006;20:1218-49. [Crossref] [PubMed]
- Ito D, Fujimoto K, Mori T, et al. In vivo antitumor effect of the mTOR inhibitor CCI-779 and gemcitabine in xenograft models of human pancreatic cancer. Int J Cancer 2006;118:2337-43. [Crossref] [PubMed]
- Center ULCC. BKM120 + mFOLFOX6 in Advanced Solid Tumors With Expansion Cohort Pancreatic Cancer.: In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT01571024, NLM Identifier: NCT01571024.
- Rexahn Pharmaceuticals I. A Safety and Efficacy Study of RX-0201 Plus Gemcitabine in Metastatic Pancreatic Cancer.: In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT01028495, NLM Identifier: NCT01028495.
- Pharmaceuticals. N. Safety, Pharmacokinetics and Pharmacodynamics of BEZ235 Plus MEK162 in Selected Advanced Solid Tumor Patients. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT01337765, NLM Identifier: NCT01337765.
- Wolpin BM, Hezel AF, Abrams T, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol 2009;27:193-8. [Crossref] [PubMed]
- Kordes S, Klumpen HJ, Weterman MJ, et al. Phase II study of capecitabine and the oral mTOR inhibitor everolimus in patients with advanced pancreatic cancer. Cancer Chemother Pharmacol 2015;75:1135-41. [Crossref] [PubMed]
- Pharmaceuticals. N. Treatment of Patients Suffering From a Progressive Pancreas Carcinoma With Everolimus (RAD001) and Gemcitabine. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT00560963, NLM Identifier:NCT00560963.
- (NCI). NCI. CCI-779 in Treating Patients With Locally Advanced or Metastatic Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT00075647, NLM Identifier: NCT00075647.
- Heinemann V, Reni M, Ychou M, et al. Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies. Cancer Treat Rev 2014;40:118-28. [Crossref] [PubMed]
- Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418-29. [Crossref] [PubMed]
- Therapeutics H. PEGPH20 Plus Nab-Paclitaxel Plus Gemcitabine Compared With Nab-Paclitaxel Plus Gemcitabine in Subjects With Stage IV Untreated Pancreatic Cancer (HALO-109-202). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://clinicaltrials.gov/ct2/show/NCT01839487, NLM Identifier: NCT01839487.
- Hwang RF, Yokoi K, Bucana CD, et al. Inhibition of platelet-derived growth factor receptor phosphorylation by STI571 (Gleevec) reduces growth and metastasis of human pancreatic carcinoma in an orthotopic nude mouse model. Clin Cancer Res 2003;9:6534-44. [PubMed]
- Institute RPC. Dovitinib Lactate, Gemcitabine Hydrochloride, and Capecitabine in Treating Patients With Advanced or Metastatic Solid Tumors, Pancreatic Cancer and Biliary Cancers. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) Available online: https://clinicaltrials.gov/ct2/show/NCT01497392, NLM Identifier: NCT01497392.
- Bramhall SR, Schulz J, Nemunaitis J, et al. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer 2002;87:161-7. [Crossref] [PubMed]
- Lichty BD, Breitbach CJ, Stojdl DF, et al. Going viral with cancer immunotherapy. Nat Rev Cancer 2014;14:559-67. [Crossref] [PubMed]
- Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther 2007;15:651-9. [PubMed]
- Naik S, Russell SJ. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther 2009;9:1163-76. [Crossref] [PubMed]
- Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res 2014;2:295-300. [Crossref] [PubMed]
- Wong HH, Lemoine NR, Wang Y. Oncolytic Viruses for Cancer Therapy: Overcoming the Obstacles. Viruses 2010;2:78-106. [Crossref] [PubMed]
- Martuza RL, Malick A, Markert JM, et al. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 1991;252:854-6. [Crossref] [PubMed]
- Thorne SH, Hwang TH, O'Gorman WE, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest 2007;117:3350-8. [Crossref] [PubMed]
- Pan W, Bodempudi V, Esfandyari T, et al. Utilizing ras signaling pathway to direct selective replication of herpes simplex virus-1. PloS One 2009;4:e6514. [Crossref] [PubMed]
- Doloff JC, Waxman DJ, Jounaidi Y. Human telomerase reverse transcriptase promoter-driven oncolytic adenovirus with E1B-19 kDa and E1B-55 kDa gene deletions. Hum Gene Ther 2008;19:1383-400. [Crossref] [PubMed]
- Nishimoto T, Yoshida K, Miura Y, et al. Oncolytic virus therapy for pancreatic cancer using the adenovirus library displaying random peptides on the fiber knob. Gene Ther 2009;16:669-80. [Crossref] [PubMed]
- Conner J, Braidwood L, Brown SM. A strategy for systemic delivery of the oncolytic herpes virus HSV1716: redirected tropism by antibody-binding sites incorporated on the virion surface as a glycoprotein D fusion protein. Gene Ther 2008;15:1579-92. [Crossref] [PubMed]
- Heinemann MK. Buy one - get one free! Thorac Cardiovasc Surg 2014;62:1-2. [Crossref] [PubMed]
- Sobol PT, Boudreau JE, Stephenson K, et al. Adaptive antiviral immunity is a determinant of the therapeutic success of oncolytic virotherapy. Mol Ther 2011;19:335-44. [Crossref] [PubMed]
- Dai MH, Zamarin D, Gao SP, et al. Synergistic action of oncolytic herpes simplex virus and radiotherapy in pancreatic cancer cell lines. Br J Surg 2010;97:1385-94. [Crossref] [PubMed]
- Pandha HS, Heinemann L, Simpson GR, et al. Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma. Clin Cancer Res 2009;15:6158-66. [Crossref] [PubMed]
- Nishizaki M, Meyn RE, Levy LB, et al. Synergistic inhibition of human lung cancer cell growth by adenovirus-mediated wild-type p53 gene transfer in combination with docetaxel and radiation therapeutics in vitro and in vivo. Clin Cancer Res 2001;7:2887-97. [PubMed]
- Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets 2007;7:141-8. [Crossref] [PubMed]
- Green NK, Seymour LW. Adenoviral vectors: systemic delivery and tumor targeting. Cancer Gene Ther 2002;9:1036-42. [Crossref] [PubMed]
- O'Shea CC, Johnson L, Bagus B, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell 2004;6:611-23. [Crossref] [PubMed]
- ClinicalTrials.gov. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); [2015]. Available online: http: //www.clinicaltrials.gov.
- Chantrill LA, Nagrial AM, Watson C, et al. Precision Medicine for Advanced Pancreas Cancer: The Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) Trial. Clin Cancer Res 2015;21:2029-37. [Crossref] [PubMed]
- Ahn J, Miura Y, Yamada N, et al. Antibody fragment-conjugated polymeric micelles incorporating platinum drugs for targeted therapy of pancreatic cancer. Biomaterials 2015;39:23-30. [Crossref] [PubMed]


