Not all right-sided hearts are the same—the importance of identifying the correct diagnosis
Case presentation
A 27-year-old white woman with a reported history of “dextrocardia” was admitted after a drug overdose. On admission she was obtunded and tachycardic, but hemodynamically stable. Heart sounds were more prominent over the right chest. Lung auscultation was normal and the rest of the exam was unremarkable. She was intubated due to decreased level of consciousness, but was successfully extubated the next day. She was eventually transferred to the psychiatry ward for further treatment.
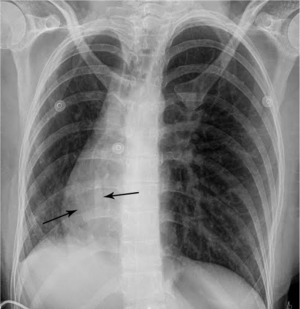
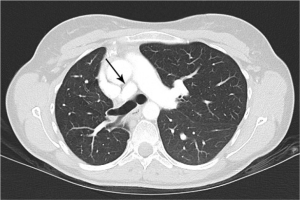
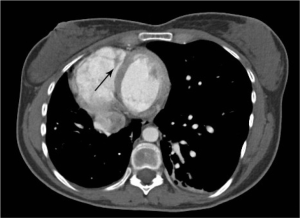
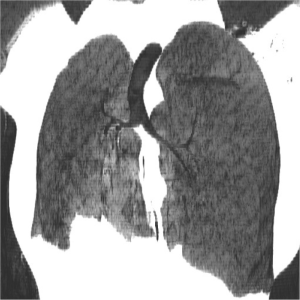
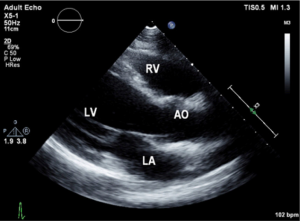
Urine toxicology screen revealed tricyclic antidepressants. Head computed tomography (CT) scan was normal. An electrocardiogram revealed sinus tachycardia. A chest roentgenogram (CxR) confirmed heart malpositioning (Figure 1). Additionally, she underwent chest CT angiography (Figures 2-5) and echocardiography (Figure 6).

At this point the differential diagnosis included Scimitar syndrome, Sawyer James syndrome, Kartagener syndrome and dextrocardia of embryonic arrest. However, closer inspection of the chest imaging revealed dextropositioning of the heart rather than dextrocardia, which essentially ruled out Kartagener syndrome and dextrocardia of embryonic arrest. Additionally, in the absence of recurrent childhood infections and presence of abnormal arterial and venous blood supply, the diagnosis of Sawyer James syndrome was not considered to be likely.
The final diagnosis was Scimitar syndrome. The CxR revealed two Scimitar veins, with chest CT angiography confirming these veins joining into a common vessel draining to the supra-hepatic inferior vena cava (IVC). Additionally, there was an aberrant systemic arterial supply to the right lower lobe, arising directly from the abdominal aorta. Other findings included a hypoplastic right pulmonary artery, hypoplasia of the right lung, and abnormal right lung lobation with a hyparterial right bronchus (left sided bronchial isomerism). These features are illustrated in Figures 2-5.
This patient was discharged home from the psychiatry ward. She was counseled regarding her diagnosis and a repeat echocardiogram and follow up visit with her primary care physician were suggested.
Discussion
Although the syndrome was first described by Chassinat and Cooper (1,2) nearly 300 years ago, the exact anatomical abnormalities were only delineated in the mid-twentieth century (3) when the term “Scimitar syndrome” was first used (4). Since then, several reports have described the corresponding anatomical abnormalities, though confusion persists regarding the classification (5,6) and management of this condition. Some include this as one of the congenital pulmonary venolobar syndromes (CPVS) (7), but many regard it as a form of partial anomalous pulmonary venous return (PAPVR) (8).
The syndrome is constantly characterized by PAPVR with oxygenated blood from the right lung draining directly into the IVC. This anomalous vein appears as a curvilinear opacity parallel to the right heart border, forming the characteristic CxR appearance of a curved sword, the “Scimitar sign”. The Scimitar syndrome is clearly differentiated from other forms of CPVS or PAPVR by this characteristic “Scimitar” shaped vein which is not present when it drains to other areas such as the superior vena cava (SVC). Associated anomalies include hypoplasia and abnormal lobation of the right lung, pulmonary sequestration, right pulmonary artery hypoplasia, heart dextroposition, anomalous systemic right lower lung arterial supply, atrial septal defect, and right diaphragmatic hernia (9).
This condition is usually diagnosed in older children during evaluation for unexplained dyspnea, but the diagnosis is seldom entertained in adults due to its rarity and lack of recognition. The incidence is reported as 1–3 in 100,000 live births (10), although the true incidence might be higher, as nearly half of the patients remain asymptomatic.
There is a correlation between presenting age and disease severity, with the disease accordingly being divided into three groups. Group 1 is the adult form with no evidence of pulmonary artery hypertension (PAH), as in our patient. Group 3 is the infantile form with severe PAH, conveying poor prognosis. The remaining patients fall into group 2 and have variable degrees of PAH and symptoms (11).
The most common radiographic findings include heart dextroposition and right sided mediastinal shift with compensatory left lung hyperinflation. However, the most pathognomonic radiographic feature remains the Scimitar sign, corresponding to the anomalous vein draining into the IVC. Therefore, the “Scimitar syndrome” term should be reserved only for cases of PAPVR draining to the IVC, despite pathophysiological commonalities with those with alternate drainage, such as to the SVC. Finally, one must understand that this finding may be occasionally obscured, due to the degree of dextroposition. In these cases, echocardiography and chest CT angiography should be performed to establish the correct diagnosis.
Echocardiography allows not only detecting the Scimitar vein behind the right atrium (12), but also identifying other commonly associated cardiac anomalies. Currently, there is increased recognition of this syndrome in-utero using echocardiography which aids in planning care for some of the complicated cases.
CT scans have been used to describe PAPVR since the 1980s. Currently, advanced technology with 3-D reconstructed images, aside from visualizing thoracic vasculature (13), provides information on the degree of left-to-right shunting (14). Furthermore, CT scans detect lung sequestration which, albeit an elusive finding, is an important one as it poses a risk for recurrent lung infections. Finally, despite all the information gathered from non-invasive studies, cardiac catheterization is still sometimes required to confirm pulmonary hypertension and/or to better define the degree of pulmonary hypertension.
Management of the Scimitar syndrome focuses on risk prevention, such as avoiding recurrent infections, with identification of sequestered lung and resection when necessary. Additionally, repair of the anomalous pulmonary venous return in symptomatic patients with moderate to severe left to right shunting and associated right ventricular strain and pulmonary hypertension is required (15). Interestingly, there is a reported case of congenitally stenotic scimitar vessel in which the scimitar vessel was stenotic at the junction of the IVC allowing for the majority of the oxygenated blood from the lung to be drained into the left atrium and hence not requiring repair of the anomalous pulmonary venous return (16). This case exemplifies the need for detailed and objective assessment of the degree of shunting, in order to better guide therapy. Experts suggest using Doppler echocardiography (17) to determine pulmonary and systemic flow ratios (Qp:Qs) estimating left to right heart shunt. A ratio of 1:1 indicates equal volumes of blood travel through the lungs (Qp) and the systemic circulation (Qs) (18). In other congenital cardiac conditions the shunt ratio helps prognosticate the need for surgery (8,19). A similar approach could be used in the Scimitar syndrome, suggesting that ratios ≥1.5 indicate consideration for corrective surgery.
Fortunately, most patients with Scimitar syndrome remain asymptomatic, leading normal lives. However, to avoid potential complications and evolving cardio-pulmonary pathology, we propose that, aside from identifying and correcting lung sequestration when needed (with recurrent lung infections), these patients should have yearly (sooner when symptomatic) echocardiography to assess for the presence and/or degree of left to right shunt (20).
Additionally, management of aberrant arterial blood supply of the sequestered lung segment is worth mentioning as patients with Scimitar syndrome often present with a sequestered lung segment. There has been a case report that details fatal complications as a result of disruption of systemic arterial supply of the intra-lobar sequestered lung segment (21). Fortunately, our patient did not present with this complication. Detailed explanation of this condition to the patient and the family can help in earlier recognition and management of this potentially fatal complication.
In conclusion, we present a case illustrating the importance of distinction between cardiac dextroposition and true dextrocardia as adequate recognition of the pathology in patients with a right sided heart allows physicians to provide better care and avoid potential complications of the condition, particularly if the diagnosis is "Scimitar syndrome".
Learning points
- Not all right sided hearts represent dextrocardia;
- Patients with heart malpositioning must have careful review of chest imaging;
- Further confirmation of Scimitar syndrome can be made through echocardiography and heart catheterization;
- Patients with Scimitar syndrome should be monitored closely for developing pulmonary hypertension and consideration for potential surgery in order to avert right heart failure;
- Sequestered lung is an important consideration when assessing for recurrent lung infections.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Cooper G. Case of malformation of the thoracic viscera consisting of imperfect development of the right lung and transposition of the heart. Lond Med Gaz 1836;18:600-1.
- Cahssinat R. Observation d'anomalies anatomiques remarquables de l'appareil circulatoire avec hepatocele congéenitale ayant donné lieu pendant la vie á aucun symptome particulier. Arch Gen Med Paris 1836;11:80.
- Halasz NA, Halloran KH, Liebow AA. Bronchial and arterial anomalies with drainage of the right lung into the inferior vena cava. Circulation 1956;14:826-46. [Crossref] [PubMed]
- Neill CA, Ferencz C, Sabiston DC, et al. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage "scimitar syndrome". Bull Johns Hopkins Hosp 1960;107:1-21. [PubMed]
- Lee ML, Lue HC, Chiu IS, et al. A systematic classification of the congenital bronchopulmonary vascular malformations: dysmorphogeneses of the primitive foregut system and the primitive aortic arch system. Yonsei Med J 2008;49:90-102. [Crossref] [PubMed]
- Irodi A, Prabhu SM, John RA, et al. Congenital bronchopulmonary vascular malformations, "sequestration" and beyond. Indian J Radiol Imaging 2015;25:35-43. [Crossref] [PubMed]
- Woodring JH, Howard TA, Kanga JF. Congenital pulmonary venolobar syndrome revisited. Radiographics 1994;14:349-69. [Crossref] [PubMed]
- Sears EH, Aliotta JM, Klinger JR. Partial anomalous pulmonary venous return presenting with adult-onset pulmonary hypertension. Pulm Circ 2012;2:250-5. [Crossref] [PubMed]
- Berrocal T, Madrid C, Novo S, et al. Congenital anomalies of the tracheobronchial tree, lung, and mediastinum: embryology, radiology, and pathology. Radiographics 2004;24:e17. [Crossref] [PubMed]
- Dupuis C, Charaf LA, Brevière GM, et al. The "adult" form of the scimitar syndrome. Am J Cardiol 1992;70:502-7. [Crossref] [PubMed]
- Espinola-Zavaleta N, Játiva-Chávez S, Muñoz-Castellanos L, et al. Clinical and echocardiographic characteristics of scimitar syndrome. Rev Esp Cardiol 2006;59:284-8. [Crossref] [PubMed]
- Valsangiacomo ER, Hornberger LK, Barrea C, et al. Partial and total anomalous pulmonary venous connection in the fetus: two-dimensional and Doppler echocardiographic findings. Ultrasound Obstet Gynecol 2003;22:257-63. [Crossref] [PubMed]
- Inoue T, Ichihara M, Uchida T, et al. Three-dimensional computed tomography showing partial anomalous pulmonary venous connection complicated by the scimitar syndrome. Circulation 2002;105:663. [Crossref] [PubMed]
- Hornero F, Canovas S, Estornell J, et al. Scimitar syndrome: multislice computer tomography with three-dimensional reconstruction. Interact Cardiovasc Thorac Surg 2003;2:341-4. [Crossref] [PubMed]
- Schramel FM, Westermann CJ, Knaepen PJ, et al. The scimitar syndrome: clinical spectrum and surgical treatment. Eur Respir J 1995;8:196-201. [Crossref] [PubMed]
- Cantinotti M, Giordano R, Spadoni I. Congenitally palliated scimitar syndrome. Cardiol Young 2015;25:1218-20. [Crossref] [PubMed]
- Slater J, Gindea AJ, Freedberg RS, et al. Comparison of cardiac catheterization and Doppler echocardiography in the decision to operate in aortic and mitral valve disease. J Am Coll Cardiol 1991;17:1026-36. [Crossref] [PubMed]
- Driscoll DJ. Left-to-right shunt lesions. Pediatr Clin North Am 1999;46:355-68. x. [Crossref] [PubMed]
- Kavga M, Varlamis G, Giannopoulos A, et al. Correlation of plasma B-type natriuretic peptide with shunt volume in children with congenital heart disease involving left-to-right shunt. Hellenic J Cardiol 2013;54:192-8. [PubMed]
- Weiman DS, Lee K, Levett JM, et al. Partial anomalous pulmonary venous return: a ten-year experience. Tex Heart Inst J 1985;12:239-43. [PubMed]
- Rubin EM, Garcia H, Horowitz MD, et al. Fatal massive hemoptysis secondary to intralobar sequestration. Chest 1994;106:954-5. [Crossref] [PubMed]


