The clinics of acute coronary syndrome
Introduction
The history of the knowledge of coronary artery disease and of the research for optimal diagnostic tools are quite longstanding (1). Probably, the very first description of ischemic chest pain was formulated around the 1550 BC, when Egyptians reported a realistic description of heart ischemia in the Ebers Papyrus, “if thou examinest a man for illness in his cardia and he has pains in his arms, and in his breast and in one side of his cardia…it is death threatening him.” (2). However, it is generally acknowledged that the first clinically acceptable description of angina pectoris is due to by William Heberden’s article published in 1772 (3). More than a century was then needed for physicians to focus their attention on coronary arteries, and to link coronary disease with chest pain. In the first issue of the New England Journal of Medicine and Surgery, published in 1812, a thorough description of angina pectoris was published by John Warren, in which the symptoms were linked to the presence of coronary disease (4). This description still retains a foremost value. Several decades later, in 1889, Ludwig Hektoen clearly demonstrated that myocardial infarction (MI) is caused by coronary thrombosis “secondary to sclerotic changes in the coronaries.” (5). Notably, the Russian clinicians and pathologists Obrastzov and Straschesko described in 1910 the cases of five patients presenting with clinical picture of MI, which was subsequently confirmed at autopsy (6).
Acute cardiac ischemic events, however, are not simply the consequence of a single problem (i.e., coronarosclerosis), but rather the result of a complex cascade of events, that have been assimilated to a perfect storm. The “perfect storm” model refers to a convergence of events and processes, encompassing formation of atherosclerotic plaque, coronary flow dynamics, hemostatic and fibrinolytic balance, metabolic and inflammatory processes, neurohormonal activation, and environmental factors that ultimately converge and trigger the ACS event (7).
Overview on chest pain
Since the times of Obrastzov and Straschesko, chest pain has become a major medical problem since it mirrors an alarm sign for cardiac ischemia and MI. At present chest pain represents one of the most common leading complaints of patients visiting an emergency department (ED) (8). It was only at the dawn of the new millennium, in the 2000, that the First Global Task Force for MI published a new definition of MI, encompassing that “any necrosis in the setting of myocardial ischemia should be labelled as MI.” (9). This definition is still valid, and has been refined in two subsequent revisions, culminating in 2012 in the third universal definition of MI (10).
Chest pain, however, is grounded on a wide spectrum of causes, ranging from totally harmless to immediately life-threatening. Amongst the latter, acute coronary syndrome [ACS, defined as a spectrum of disease ranging from unstable angina (UA) to MI], pulmonary embolism, aortic dissection, tension pneumothorax, pericardial tamponade, and esophageal rupture are the bogeymans of the emergency physicians (EPs), that have the constant concern and duty to rule out potentially life-threatening causes and to act a diagnostic and management strategy allowing a rapid and safe disposition of patients. ACS, however, only accounts for 20–25% of chest pain patients visited in ED (11), and only for 45% of patients admitted to a Chest Pain Unit (12).
A myriad of other causes of chest pain have been recognized, including pneumonia, anxiety, migraine, musculoskeletal, skin and gastrointestinal ones (see Table 1). Notably, in a significant percentage of patients, the cause of chest pain is never found. In the last decades several studies showed a worrisome high rate (between 2–4%) of patients with missed ACS who were discharged from the ED (13,14). Unfortunately, the patients incorrectly discharged have a higher short-term mortality, ranging between 10% and 25% (13,14).
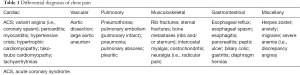
Full table
This evidence has contributed to rise the EPs’ fear of being sued for missed ACS, thus leading to order a constantly increasing number of tests and causing many inappropriate admissions, even for patients with low-risk chest pain (15). On the other hand, the increasing overcrowding of the EDs is posing serious threats to the health care systems around the globe, thus raising additional concern about the safety and overall efficiency of the emergency systems (16). ED’s overcrowding has been clearly linked with increased mortality, medication errors, pain and length of stay (17), most notably in chest pain patients (18), thus generating a dog chasing its own tail vicious circle. As such, a rational approach, balancing risks and benefits, is urgently needed.
Value of history
The vast majority of physicians still operate with the robust certainty that the patient with ACS is classically a white man, older than 60 years, with multiple risk factors, complaining for left-sided chest pressure radiating to the arm with some combination of associated dyspnea, nausea, lightheadedness, or diaphoresis. The analysis of the clinical characteristics of patients erroneously discharged from EDs with missed ACS, however, is quite different. It describes a non-white subject, younger, less likely to have a history of known coronary artery disease (CAD), and less likely to identify chest pain as his chief complaint patients (13,14). Different studies demonstrated that typical symptoms are useful diagnostic tools in patients with stable coronary artery disease (19-21), but the same does not hold true in patients presenting with chest pain in the ED (22-26). One additional risk factor for missing the diagnosis of ACS has been recently described, consisting in ED overall volume, wherein the rate of missed MI is 2-fold higher in low-volume facilities (ranging from 0% in the EDs with the highest volume of patients to a troubling 29% at one low-volume facility, respectively) (27).
In the past, the gender of the patients was emphasized as a strong factor associated with differences in clinical presentation of ACS. Recent data, however, seems to refute this belief, showing that differences in the sex-specific diagnostic performance of clinical prediction rules are modest and not seemingly support the use of women-specific rules in the early diagnosis of MI (25).
In the US, African Americans visited in the ED with chest pain tend to be younger, are more likely female, and are less likely to have had a history of CAD than their white counterparts. The aforementioned features may somewhat induce EPs to a lower threshold of suspicion for ACS. Moreover, their ECGs are more frequently featured by left ventricular hypertrophy, presumably due to a higher prevalence of hypertension in this population (28).
Typicality of chest pain
After that said, since the information about age, sex, race and history of patients can be easily collected, a pivotal question remains: how should we define atypical symptoms? It seems better to start from typical symptoms. Many physicians still rely on the original Heberden’s description of angina pectoris, describing left sided substernal pain as a strangling sensation worsened by exertion and relieved by rest, that radiated to the left arm (3). This description is widely accepted and cited in articles and textbooks (22,29), as well as incorporated in several clinical prediction rules (30). Since a number of studies contributed to a progressive refinement in the accepted description of ischemic chest pain, the description is more comprehensive thus far, as reported in the Guidelines of The National Heart Attack Alert Program Coordinating Committee on recognition of symptoms potentially associated with cardiac ischemia (31). These Guidelines describe the presentation as follows: pain, if present, is described as pressure, tightness, or heaviness. It may radiate to the neck, jaw, shoulders, back, or one or both arms. The pain may also be described as indigestion or heartburn with associated nausea and/or vomiting. Additional symptoms in the absence of pain may include shortness of breath, weakness, dizziness, lightheadedness, or loss of consciousness (31). It is easy to understand that the aforementioned description, albeit capable to warrant constant attention of the EPs to the vast majority (but not all) of clinical presentations of ACS, also represents a compelling caveat to perform intensive testing in a wide subset of the whole ED population. Notably, the first sentence of the definition stats pain, if present,…. If present represents a sentence opening whole world: the obscure world of the ACS presentations without chest pain.
Regrettably, a large and thorough review aimed to analyse the value of chest pain characteristics in predicting ACS found scarce and flimsy evidence, thus concluding that although certain elements of the chest pain history are associated with increased or decreased likelihoods of a diagnosis of ACS or AMI, none of them alone or in combination identify a group of patients that can be safely discharged without further diagnostic testing (22).
Recently a comprehensive review has suggested that the more predictive characteristics for ischemic pain include pain radiation to both arms, pain similar to prior ischemia, and change in pain pattern over the prior 24 hours. Amongst the physical findings, hypotension (i.e., SBP <100 mmHg) was the strongest predictor of ACS. Data are summarized in Table 2 (32).
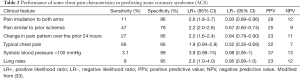
Full table
Since up to 80% of patients report more than one symptom, and some studies describe an average of 7–8 symptoms (33-35), there has been increasing interest in depicting symptom clusters in ACS, albeit with inconclusive results (36). Typicality or non-typicality of chest pain characteristics are summarized in Figure 1.
The uncertainty of the definition of the clinical presentation has led some Authors to a call for action to standardize symptom presentation in ACS (37), but this achievement is still lacking. Some think that, at least in the last decades, typical chest pain is actually atypical.
Atypical presentations and value of associated symptoms
Sweating (or diaphoresis) is often cited as one of the most frequent presentation symptoms of ACS, maybe being a signal of the activation of the sympathetic nervous system, but only recently this clinical manifestation has been extensively evaluated. In a large cohort of more than 12,000 ACS patients, the presence of sweating, in association with other ACS symptoms, well predicted the probability of STEMI [odds ratio (OR): 97.06, 95% confidence interval (CI): 82.16–114.14, P<0.0001]. For diagnosis of STEMI, the positive likelihood ratio (LR) and positive predictive value (PPV) were the highest for typical chest pain associated with sweating (LR: 11.2, 95% CI: 10.3–12.1; PPV: 76.1, 95% CI: 74.4–77.8) (38). As such, sweating in association with typical or atypical angina was found to be a good predictor of ST elevation MI (STEMI), rather than NSTE-ACS, thus confirming his nature of “red flag” for prompt evaluation and treatment of chest pain patients.
During the past few decades, a shift has been observed in the clinical presentation of ACS to milder forms, with some evidence that both case severity and mortality may actually be decreasing (39).
Another confounding factor is represented by the age of the patients. Although an excess 80% of patients who die from CAD are aged >65 years (39), the clinical presentation of ACS in elderly is even more intriguing, due to an increasing number of vague and atypical symptoms and the wide spectum of co-morbidities and accompanying complaints that are usually present in this patient group. Up to 50% of these patients show atypical presentation, most commonly dyspnea, nausea, diaphoresis, syncope, or pain primarily localized to the arm, neck, jaw, or abdomen, rather than in the chest (40,41). Moreover, it has been clearly demonstrated that, at least in Europe, elderly ACS patients are less likely to present with ST-elevation and, despite their substantial in-hospital mortality, they are definitely less intensively treated and investigated (42,43).
Effects of drugs and comorbidities
Another concern is represented by those patients who are already using nitrates. In a large subset of the GRACE registry it was found that patients chronically using nitrates are more prone to develop NSTE-ACS than to develop STEMI, and usually show a less release of markers of cardiac necrosis (44). These findings suggest that acute coronary events may develop to a smaller extent in nitrate users, maybe due to a mechanism similar to the so called preconditioning phenomenon (i.e., brief episodes of ischemia increase the tolerance of the heart to a subsequent major ischemic insult). Several Authors believe that the preconditioning phenomenon is linked to the sequence of events represented by activation of nitric oxide (NO) synthase, with its attendant production of NO (45), that could be mimicked by chronic nitrates use.
One relatively new subset of chest pain patients is represented by human immunodeficiency virus (HIV)-infected patients. It has recently been demonstrated that these patients are at higher risk for ACS (46). A recent report showed that HIV-infected patients were younger, more frequently men, complained for fewer and milder symptoms, and had higher prevalence of cardiovascular risk factors than non-infected patients. Notably, 11% of HIV-infected patients had a history of recent use of cocaine or other abuse substances. ST-elevation MI was the most frequent ACS in HIV-infected patients (59% vs. 24%) whereas non-ST-elevation MI (23% vs. 38%) and UA (18% vs. 38%) were more predominant in uninfected patients (P<0.001) (47).
Being married is generally thought to be a good and healthy condition (48), and even in the field of ACS this belief seems to be confirmed. In fact, at least in men, being married was associated with significantly earlier presentation for care, a benefit that was not observed for married women. As such, earlier presentation for medical care appears to be one reason for the observed lower risk of cardiovascular death among married men, relative to their single counterparts (49).
Asymptomatic myocardial infarctions (MIs)
In the Framingham study, representing the greater and most studied group of patients in history, up to 25% of patients were found to have a Q-wave MI on routine annual ECGs, in absence of any previous clinical manifestation, so exhibiting truly silent MIs (50). More recently, a study using delayed enhancement cardiovascular magnetic resonance imaging in high-risk patients discovered an incidence of unrecognized non-Q-wave MIs approaching a 3-fold higher frequency than unrecognized Q-wave ones (51). Since we know now that non-Q-wave MIs are much more common than Q-wave MIs, these data represent an astonishing number of silent, unrecognized MIs (52,53). A pivotal study conducted on more than 434,800 patients included in the National Registry of Myocardial Infarction 2 database (NRMI-2) with a diagnosis of MI reported that 33% of them did not have chest discomfort or arm, neck, or jaw pain on initial presentation to the hospital. MI patients presenting without chest pain were, on average, seven years older and showed a higher prevalence of women and diabetics than those presenting with chest pain (54). A study of the Global Registry of Coronary Events (GRACE), including more than 20,000 cases of ACS, found that 8.4% of them did not have chest pain at presentation, and in almost a quarter of these the diagnosis of ACS was missed on their initial evaluation. The most common symptom in the patients presenting without chest pain was dyspnea, followed by diaphoresis, nausea, and syncope. In this study the absence of chest pain was also more frequent in elderly, female, hypertensive, diabetic, or in those with a history of congestive heart failure. Notably, the mortality was much higher in all the subgroups of patients presenting without chest pain, being the highest in patients presenting with syncope (55). As such, the absence of chest pain seems to be the greatest pitfall for the EPs, and one of the worst predictors of mortality.
Precipitating and relieving factors
The EPs usually ask the patients in which circumstances the chest pain occurred. A meta-analysis of 17 studies including more than 10,000 MI patients showed that 35% were engaged in physical activity, 20% were awakened from sleep, 8.2% were eating and 6.8% reported emotional stress just before the event (56). Therefore, the take home message is: do not underrate emotional stress or eating as precipitating factors, considering them as symptoms of anxiety or digestive disease.
In parallel, some relieving factors are poor indicators for cardiac or non-cardiac origin of chest pain. This is especially true for nitroglycerine and antacids. In particular, chest-pain relief by nitroglycerine had no value for ruling-in or ruling-out ACS, showing a higher incidence of relief in patients without ACS, also relieving chest pain in 66% of patients with noncardiac chest pain (57,58). Similarly, when using antacids or the so-called gastrointestinal cocktail (a mix of antacids and lidocaine), a significant portion of patients with myocardial ischemia reported total or partial relief (59). So, nor nitroglycerine and neither gastrointestinal cocktail should be used to influence diagnostic process in chest pain patients.
Clinical gestalt versus structured scores
What is the value of the initial clinical impression and judgement of the EPs? In other words: how much can the clinicians rely on their Gestalt perception about clinical presentation of chest pain patients? It has been shown, in a group of 840 patients with chest pain and/or dyspnea, that clinical Gestalt produced higher estimates of likelihood of ACS (17% versus 4%) and pulmonary embolism (12% versus 6%) in a relatively low-risk population (2.7% ACS; 1.8% pulmonary embolism) (60). Another well-performed study analyzed the diagnostic accuracy of overall clinical impression (i.e., physicians estimated the probability of ACS before knowing troponin values and ECG results, but after taking a full history) in the diagnosis of ACS. A choice of “definite” ACS had a diagnostic LR of 4.0 (95% CI, 2.5–6.6); “probable” ACS, 1.8 (95% CI, 1.3–2.4); “could be” ACS, 0.66 (95% CI, 0.46–0.96); “probably not” ACS, 0.20 (95% CI, 0.09–0.44); and “definitely not” ACS, 0.36 (95% CI, 0.05–2.8) (61). A recent and well performed Swedish study showed that: (I) in patients aged less than 40 years, chest pain history and overall Gestalt not suspicious of ACS were of high value in ruling out virtually all ACS; (II) a positive initial troponin value and an ischemic ECG were strong predictors of ACS, seemingly superior to pain history for ruling in ACS; (III) in patients with a normal initial troponin value and non-ischemic ECG, a chest pain history typical of MI was not a significant predictor of MI, while chest pain history typical of UA was a moderate predictor of UA. Therefore, the Authors concluded that Gestalt was better than its components both for ruling in (LR 29) and ruling out (LR 0.01) ACS (62). Moreover, emerging evidence seems to demonstrate that EPs with greater experience are less likely to miss ACS, being the average number of years of ED experience 2.6 for those missing the diagnosis versus 5.1 for controls, respectively (63). As such, the younger physicians, although spontaneously oriented toward an intuitive and heuristic thought, need to perform an analytical and structured evaluation first, followed by a Gestalt perception of the whole aspect of the problem. On the contrary, the skilled, expert physicians, after incorporating a number of ‘‘spontaneous flowcharts’’ in their personal toolbox, seem to develop a natural and spontaneous inclination to Gestalt perception of patient’s problems first, followed (when needed) by accurate systematic analysis of the single aspects of the clinical picture. Probably, it seems reasonable to put forward the hypothesis that a wise synthesis of Gestalt perception, Bayesians principles, and technology should be performed in every field of medicine, in particular in those fields like emergency medicine in which the time is limited (64).
Conclusions
Almost a century after the pioneering observations of the ECG abnormalities associated with experimental coronary artery ligation in dogs (65), and the pivotal description, by Harold Pardee of the ECG changes associated with MI in humans (i.e., the early rise of ST-segment along with the takeoff of the T-wave from the descending R-wave during the early phase of myocardial ischemia: the “Pardee’s (or, sometimes, Smith-Pardee’s) sign) (66), the 12-lead ECG continues to be the single most important and most rapidly available diagnostic test used in the management of the patient with chest pain. However, the revision of initial ECG recordings of 391,208 patients with documented AMI, in the NRMI database, showed that 7.8% had a normal ECG recording and 35.2% had nonspecific ECG findings (67). As such, every EP should keep in mind that a normal or nonspecific ECG finding cannot, per se, be used to rule out cardiac ischemia or MI.
On the other side, the EPs should also keep in mind that hospital admission of patients with chest pain with two negative findings for serial biomarkers, nonconcerning vital signs, and nonischemic ECG findings, can induce iatrogenic short-term clinically relevant adverse events, thus suggesting that routine inpatient admission may not be a beneficial strategy for this group (68).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
References
- Cervellin G, Lippi G. Of MIs and Men. A Historical Perspective on the Diagnostics of Acute Myocardial Infarction. Semin Thromb Hemost 2014;40:535-43. [Crossref] [PubMed]
- Boisaubin EV. Cardiology in ancient Egypt. Tex Heart Inst J 1988;15:80-5. [PubMed]
- Heberden W. Some account of a disorder of the breast. Medical Transactions 1772;2:59-67.
- Warren J. Remarks on angina pectoris. N Engl J Med Surg 1812;1:1-11.
- Hektoen L. Embolism of the left coronary artery; sudden death. Med Newsl (Lond) 1892;61:210.
- Obrastzow WP, Straschesko ND. Zur Kenntnis der Thrombose der Koronararterien des Herzens. Z Klin Med 1910;71:116-32.
- Burg MM, Edmondson D, Shimbo D, et al. The ‘Perfect Storm’ and Acute Coronary Syndrome Onset: Do Psychosocial Factors Play a Role? Prog Cardiovasc Dis 2013;55:601-10. [Crossref] [PubMed]
- Pitts SR, Niska RW, Xu J, et al. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Report 2008;7:1-38. [PubMed]
- Alpert JS, Thygesen K, Antman E, et al. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959-69. [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020-35. [Crossref] [PubMed]
- Goodacre S, Cross E, Arnold J, et al. The health care burden of acute chest pain. Heart. 2005;91:229-30. [Crossref] [PubMed]
- Conti A, Paladini B, Toccafondi S, et al. Effectiveness of a multidisciplinary chest pain unit for the assessment of coronary syndromes and risk stratification in the Florence area. Am Heart J 2002;144:630-5. [Crossref] [PubMed]
- Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163-70. [Crossref] [PubMed]
- McCarthy BD, Beshansky JR, D’Agostino RB, et al. Missed diagnoses of acute myocardial infarction in the emergency department: results from a multicenter study. Ann Emerg Med 1993;22:579-82. [Crossref] [PubMed]
- Katz DA, Williams GC, Brown RL, et al. Emergency physicians’ fear of malpractice in evaluating patients with possible acute cardiac ischemia. Ann Emerg Med 2005;46:525-33. [Crossref] [PubMed]
- Derlet RW, McNamara RM, Kazzi AA, et al. Emergency department crowding and loss of medical licensure: a new risk of patient care in hallways. West J Emerg Med 2014;15:137-41. [Crossref] [PubMed]
- McCusker J, Vadeboncoeur A, Lévesque JF, et al. Increases in Emergency Department Occupancy Are Associated With Adverse 30-day Outcomes Acad Emerg Med 2014;21:1092-100. [Crossref] [PubMed]
- Diercks DB, Roe MT, Chen AY, et al. Prolonged emergency department stays of non-ST-segment-elevation myocardial infarction patients are associated with worse adherence to the American College of Cardiology/American Heart Association guidelines for management and increased adverse events. Ann Emerg Med 2007;50:489-96. [Crossref] [PubMed]
- Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979;300:1350-8. [Crossref] [PubMed]
- Pryor DB, Shaw L, McCants CB, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med 1993;118:81-90. [Crossref] [PubMed]
- Genders TS, Steyerberg EW, Alkadhi H, et al. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J 2011;32:1316-30. [Crossref] [PubMed]
- Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA 2005;294:2623-9. [Crossref] [PubMed]
- Goodacre S, Locker T, Morris F, et al. How useful are clinical features in the diagnosis of acute undifferentiated chest pain? Acad Emerg Med 2002;9:203-8. [Crossref] [PubMed]
- Body R, Carley S, Wibberley C, et al. The value of symptoms and signs in the emergent diagnosis of acute coronary syndromes. Resuscitation 2010;81:281-6. [Crossref] [PubMed]
- Rubini Gimenez M, Reiter M, Twerenbold R, et al. Sex-specific chest pain characteristics in the early diagnosis of acute myocardial infarction. JAMA Intern Med 2014;174:241-9. [Crossref] [PubMed]
- Carlton EW, Than M, Cullen L, et al. ‘Chest Pain Typicality’ in Suspected Acute Coronary Syndromes and the Impact of Clinical Experience. Amer J Med 2015;128:1109-1116.e2. [Crossref] [PubMed]
- Schull MJ, Vermeulen MJ, Stukel TA. The risk of missed diagnosis of acute myocardial infarction associated with emergency department volume. Ann Emerg Med 2006;48:647-55. [Crossref] [PubMed]
- Johnson PA, Lee TH, Cook EF, et al. Effect of race on the presentation and management of patients with acute chest pain. Ann Intern Med 1993;118:593-601. [Crossref] [PubMed]
- Goodacre SW, Angelini K, Arnold J, et al. Clinical predictors of acute coronary syndromes in patients with undifferentiated chest pain. QJM 2003;96:893-8. [Crossref] [PubMed]
- Hess EP, Thiruganasambandamoorthy V, Wells GA, et al. Diagnostic accuracy of clinical prediction rules to exclude acute coronary syndrome in the emergency department setting: a systematic review. CJEM 2008;10:373-82. [PubMed]
- Emergency department: rapid identification and treatment of patients with acute myocardial infarction. National Heart Attack Alert Program Coordinating Committee, 60 Minutes to Treatment Working Group. Ann Emerg Med 1994;23:311-29. [Crossref] [PubMed]
- Fanaroff AC, Rymer JA, Goldstein SA, et al. Does This Patient With Chest Pain Have Acute Coronary Syndrome? The Rational Clinical Examination Systematic Review. JAMA 2015;314:1955-65. [Crossref] [PubMed]
- Richards SB, Funk M, Milner KA. Differences between Blacks and Whites with coronary heart disease in initial symptoms and in delay in seeking care. Am J Crit Care 2000;9:237-44. [PubMed]
- McSweeney JC, Cody M, O’Sullivan P, et al. Women’s early warning symptoms of acute myocardial infarction. Circulation 2003;108:2619-23. [Crossref] [PubMed]
- DeVon HA, Ryan CJ, Ochs AL, et al. Symptoms across the continuum of acute coronary syndromes: differences between women and men. Am J Crit Care 2008;17:14-24. [PubMed]
- Rosenfeld AG, Knight EP, Steffen A, et al. Symptom clusters in patients presenting to the emergency department with possible acute coronary syndrome differ by sex, age, and discharge diagnosis. Heart & Lung 2015;44:368-75. [Crossref] [PubMed]
- Canto JG, Goldberg RJ, Sopko G. A call to standardize symptom presentation in acute coronary syndromes. Am Heart J 2012;164:801-6. [Crossref] [PubMed]
- Gokhroo RK, Ranwa BL, Kishor K, et al. Sweating: A Specific Predictor of ST-Segment Elevation Myocardial Infarction Among the Symptoms of Acute Coronary Syndrome: Sweating In Myocardial Infarction (SWIMI) Study Group. Clin Cardiol 2016;39:90-5. [Crossref] [PubMed]
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2016 Update. A Report From the American Heart Association. Circulation 2016;133:e38-60. [Crossref] [PubMed]
- Canto JG, Fincher C, Kiefe CI, et al. Atypical presentations among Medicare beneficiaries with unstable angina pectoris. Am J Cardiol 2002;90:248-53. [Crossref] [PubMed]
- Alexander KP, Roe MT, Chen AY, et al. Evolution in cardiovascular care for elderly patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol 2005;46:1479-87. [Crossref] [PubMed]
- Rosengren A, Wallentin L, Simoons M, et al. Age, clinical presentation, and outcome of acute coronary syndromes in the Euroheart acute coronary syndrome survey. Eur Heart J 2006;27:789-95. [Crossref] [PubMed]
- Donataccio MP, Puymirat E, Vassanelli C, et al. Presentation and revascularization patterns of patients admitted for acute coronary syndromes in France between 2004 and 2008 (from the National Observational Study of Diagnostic and Interventional Cardiac Catheterization [ONACI]). Am J Cardiol 2014;113:243-8. [Crossref] [PubMed]
- Ambrosio G, Del Pinto M, Tritto I, et al. Chronic nitrate therapy is associated with different presentation and evolution of acute coronary syndromes: insights from 52,693 patients in the Global Registry of Acute Coronary Events. Eur Heart J 2010;31:430-8. [Crossref] [PubMed]
- Cohen MV, Yang XM, Downey JM. Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res 2006;70:231-9. [Crossref] [PubMed]
- Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506-12. [Crossref] [PubMed]
- Perelló R, Calvo M, Miró O, et al. Clinical presentation of acute coronary syndrome in HIV infected adults: a retrospective analysis of a prospectively collected cohort. Eur J Intern Med 2011;22:485-8. [Crossref] [PubMed]
- Ben-Shlomo Y, Smith GD, Shipley M, et al. Magnitude and causes of mortality differences between married and unmarried men. J Epidemiol Community Health 1993;47:200-5. [Crossref] [PubMed]
- Atzema CL, Austin PC, Huynh T, et al. Effect of marriage on duration of chest pain associated with acute myocardial infarction before seeking care. CMAJ 2011;183:1482-91. [Crossref] [PubMed]
- Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. An update on the Framingham study. N Engl J Med 1984;311:1144-7. [Crossref] [PubMed]
- Kim HW, Klem I, Shah DJ, et al. Unrecognized non-Q-wave myocardial infarction: prevalence and prognostic significance in patients with suspected coronary disease. PLoS Med 2009;6:e1000057. [Crossref] [PubMed]
- Nikus K, Pahlm O, Wagner G, et al. Electrocardiographic classification of acute coronary syndromes: a review by a committee of the International Society for Holter and Non-Invasive Electrocardiology. J Electrocardiol 2010;43:91-103. [Crossref] [PubMed]
- Dauerman HL, Lessard D, Yarzebski J, et al. Ten-year trends in the incidence, treatment, and outcome of Q-wave myocardial infarction. Am J Cardiol 2000;86:730-5. [Crossref] [PubMed]
- Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA 2000;283:3223-9. [Crossref] [PubMed]
- Brieger D, Eagle KA, Goodman SG, et al. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: insights from the global registry of acute coronary events. Chest 2004;126:461-9. [Crossref] [PubMed]
- Culić V. Acute risk factors for myocardial infarction. Int J Cardiol 2007;117:260-9. [Crossref] [PubMed]
- Henrikson CA, Howell EE, Bush DE, et al. Chest pain relief by nitroglycerin does not predict active coronary artery disease. Ann Intern Med 2003;139:979-86. [Crossref] [PubMed]
- Grailey K, Glasziou PP. Diagnostic accuracy of nitroglycerine as a ‘test of treatment’ for cardiac chest pain: a systematic review. Emerg Med J 2012;29:173-6. [Crossref] [PubMed]
- Wrenn K, Slovis CM, Gongaware J. Using the ‘‘GI cocktail’’: a descriptive study. Ann Emerg Med 1995;26:687-90. [Crossref] [PubMed]
- Kline JA, Stubblefield WB. Clinician Gestalt Estimate of Pretest Probability for Acute Coronary Syndrome and Pulmonary Embolism in Patients With Chest Pain and Dyspnea. Ann Emerg Med 2014;63:275-80. [Crossref] [PubMed]
- Body R, Cook G, Burrows G, et al. Can emergency physicians “rule in” and “rule out” acute myocardial infarction with clinical judgement? Emerg Med J 2014;31:872-6. [Crossref] [PubMed]
- Mokhtari A, Dryver E, Söderholm M, et al. Diagnostic values of chest pain history, ECG, troponin and clinical gestalt in patients with chest pain and potential acute coronary syndrome assessed in the emergency department. SpringerPlus 2015;4:219. [Crossref] [PubMed]
- Rusnak RA, Stair TO, Hansen K, et al. Litigation against the emergency physician: common features in cases of missed myocardial infarction. Ann Emerg Med 1989;18:1029-34. [Crossref] [PubMed]
- Cervellin G, Borghi L, Lippi G. Do clinicians decide relying primarily on Bayesians principles or on Gestalt perception? Some pearls and pitfalls of Gestalt perception in medicine. Intern Emerg Med 2014;9:513-9. [Crossref] [PubMed]
- Smith FM. The ligation of coronary arteries with electrocardiographic study. Arch Intern Med 1918;22:8-27. [Crossref]
- Pardee HE. An electrocardiographic sign of coronary artery obstruction. Arch Intern Med 1920;26:244-57. [Crossref]
- Welch RD, Zalenski RJ, Frederick PD, et al. Prognostic value of a normal or nonspecific initial electrocardiogram in acute myocardial infarction. JAMA 2001;286:1977-84. [Crossref] [PubMed]
- Weinstock MB, Weingart S, Orth F, et al. Risk for clinically relevant adverse cardiac events in patients with chest pain at hospital admission. JAMA Intern Med 2015;175:1207-12. [Crossref] [PubMed]


