Diagnostic algorithms for acute coronary syndrome—is one better than another?
Introduction
Chest pain, one of the most frequent symptoms leading patients to present to the emergency department (ED), can be triggered by a wide spectrum of causes, which span from totally harmless to immediately life-threatening triggers. In the emergency physicians (EPs) perspective, the rapid identification of high risk patients and the concomitant rule out of low risk conditions is of pivotal importance. An underlying acute coronary syndrome (ACS) accounts for approximately 20–25% of chest pain patients visited in ED (1), and for nearly 45% of those admitted to a chest pain unit (2). The leading aspects in the EPs’ toolbox that can help establishing the likelihood of ACS include patient history, electrocardiogram (ECG), and cardiac troponin(s) testing. Although a number of diagnostic algorithms have been developed and used so far, mainly designed for rapid ruling-in or ruling-out of ACS in patients with chest pain, a definitive and universally agreed strategy is still far from being identified and universally acknowledged.
The value of clinical presentation has been extensively discussed in a separate article of this issue (3). ECG has played a pivotal and almost unquestionable role for decades, but it unfortunately lacks both sensitivity and specificity, with some notable exceptions (4,5). In recent years, it has been demonstrated that the assessment of patient history and ECG can be helpful to reliably predict 30-day major adverse cardiac events (MACE) risk, but cannot safely identify those patients who could be safely discharged (6,7).
The search for a new mainstay for diagnosing and even for prognostication of patients with ischemic heart disease has led to the discovery and introduction into clinical practice of a broad array of continuously improving biomarkers (8). The first pivotal definition of myocardial infarction (MI), that represents only one amongst the different clinical manifestations of ACSs, has been released in the early 1970s by the World Health Organization (WHO). The first document, which was published in 1976 and conventionally known as “European Myocardial Infarction registry criteria”, established that the diagnosis of MI could be made on the basis of clinical history, ECG findings, cardiac enzymes testing and postmortem findings (9). Indeed, the role of cardiac biomarkers was rather limited at that time, since the available tests [i.e., aspartate aminotransferase (AST), lactate dehydrogenase (LDH) or creatine kinase (CK)] were characterized by a poor cardiac specificity, but also by a kinetic of post-MI release that was generally unsuitable for early diagnosis of irreversible myocardial injury. A substantial improvement occurred in 1973, with the development and introduction of innovative techniques for the measurement of the isoenzyme MB of CK (i.e., CK-MB) (10) and, decades afterward, with the development of commercial methods for the measurement of serum or plasma myoglobin (11). However, it was only at the dawn of the third millennium that a major revolution occurred, with the development of monoclonal antibodies capable of specifically recognizing the cardiac isoforms of both troponins I and T (12). Shortly afterward, the consensus document published by the European Society of Cardiology/American College of Cardiology (ESC/ACC) committee for the redefinition of MI first introduced the concept that MI can be diagnosed in presence of a typical rise (or gradual fall) of cardiac troponin I or T, without the need to perform additional laboratory investigations (13). This criterion, reiterated and refined in two additional documents (14,15), is still valid and widely applied around the globe.
The development of a new generation of cardiac troponin immunoassays, conventionally defined as “highly sensitive” (HS), has subsequently represented a further analytical refinement for the measurement of this biomarker, which allowed to identify minor increases of troponin concentration at an earlier stage after the onset of cardiac symptoms and, especially, to shorten the time necessary for serial sampling, which is still recommended for identifying the highly suggestive increase that typically characterizes myocardial injury (16). Indeed, the introduction of these HS immunoassays should be regarded as a paradigm shift in the diagnostic approach of patients with chest pain (17), provided that appropriate cut-offs and reliable diagnostic algorithms can be developed and clinically validated.
In search for the perfect algorithm
After the introduction of the new HS troponin assays, several studies were planned to establish innovative (“ever shorter”) algorithms, entailing closer times for serial sampling of cardiac troponins, allowing early discharge and ultimately preventing overcrowding in short stay units. The recent literature proposes a myriad of articles recommending shortened timing for rule in and rule out of ACS, from 6 h, to 3 h and even down to 2 h (18-21). This spasmodic search has ended up with some intriguing articles published by Reichlin et al. (22,23), which recommended the use of diagnostic algorithms based on HS cardiac troponin testing at ED presentation and 1 h thereafter.
Although the 1-h approach is somehow intriguing and attractive in the ED environment, yet many questions remain unanswered. The implementation of such a limited time interval for blood sampling seems rather critical in most healthcare organizations. Although clinicians and laboratory professionals continue to support a goal for turnaround time (TAT) <60 min for obtaining results of cardiac biomarkers testing, the vast majority of data published so far demonstrated that this target cannot be met in many facilities, especially those where the clinical laboratory is located quite far from the ED (24). Therefore, the use of 1-h delta seems rather unrealistic, provided that (I) the ED and the clinical laboratory are very close, or else connected by efficient systems for sample transportation (25); (II) the ED is equipped with reliable point of care (POC) instrumentation for cardiac troponin testing (26); and (III) a HS assay is used either in the laboratory or in the ED by means of POC devices (27). Notably, the availability of cardiac troponin tests within 1 h from sample collection is also of questionable clinical usefulness, since the current guidelines recommend early invasive strategies within 24 h, whereas 2 h revascularization is only recommend in selected cases, identified according to well established clinical criteria (28). It is hence quite surprising that the 1-h algorithm, that has not received extensive clinical validation so far to the best of our knowledge, was referred to as class I recommendation in the guidelines of the ESC (28). Ironically, the date of publication of these guidelines (i.e., September, 11th) may hence be seen as “the tomb of cardiologists”, wherein the amount of information generated so far about the different timing and interpretation of cardiac troponin testing has now become virtually unmanageable for many. Surprisingly, the guidelines also report that “… the performance of the 1 h algorithm to rule in and rule out acute MI in patients presenting with chest pain to the emergency department has not been tested within a randomized controlled trial. The best management of patients assigned to the ‘observational zone’ according to the 1 h algorithm remains to be defined”, and “in patients presenting very early (e.g., within 1 h from chest pain onset), the second cardiac troponin level should be obtained at 3 h, due to the time dependency of troponin release”.
One essential criticism lays in the fact that the 1 h algorithm for patient disposition is based on troponin measurement alone. In clinical practice, however, the decision making is based on the entire clinical picture, which entails patient history, differential diagnosis, results of cardiac troponin testing and serial ECGs. In fact, in partial contradiction, the ESC Guidelines conclude that the algorithm should always be used together with an assessment of patient history and ECG (28).
As an additional pivotal issue, we should consider that a clinically acceptable diagnostic algorithm for ED patients with chest pain should be aimed to identify those patients whose risk of ACS is below the test threshold at which patients are more likely to be harmed than to get benefit from further testing (29). Recently, some studies suggested that the majority of EPs are prone to accept a <1% risk of 30-day MACE in chest pain discharged patients (30).
As a consequence of such a strenuous search of effectiveness, efficiency and safety, a combined algorithm (i.e., combining patient’s history, ECG and cardiac troponin testing) has been recently proposed (31). In brief, the Authors suggest rapid rule out when at admission (i.e., 0 h) HS-cardiac troponin T (cTnT) is <12 ng/L, and 1h post admission HS-cTnT shows a delta <3 ng/L, and a non-ischemic ECG has been recorded, and the patient history does not suggest a high risk. Conversely, a rapid rule in is suggested when at admission (i.e., 0 h) HS-cTnT is ≥52 ng/L, or 1 h post admission HS-cTnT shows a delta ≥5 ng/L, or at 0 or 1 h HS-cTnT >14 ng/L combined with either: ischemic ECG or high risk patient history.
When this algorithm has been followed, 60% of patients have been ruled out. Patients discharged had a 0.5% risk of MACE within 30 days, and almost no risk of MACE without ACS. The algorithm only missed three patients with unstable angina. At variance, 14% of patients directly ruled in had a 30-day risk of MACE of 62%, and a 30-day risk of MACE without ACS of 53%. Approximately one fourth of the whole patient cohort remained in an “observation zone”, requiring further testing, i.e., additional troponin testing and/or stress testing and/or cardiac imaging when the diagnosis remained unclear.
Obviously, when the 1-h TAT for cardiac troponin testing is not locally available, it would be reasonable to apply the same algorithm, thus implementing a 3-h interval blood sampling. It is reasonable to believe that sensitivity will not decrease, and specificity would increase, at a very low price (i.e., only 1–2 h length of stay in the ED).
According with this assumption, a couple of years ago an Italian study, endorses by the national Academy of Emergency Medicine and Care (AcEMC), the national Society of Clinical Biochemistry and Laboratory Medicine (SIBioC) and many cardiologists, proposed distinct algorithms based on the use of HS or conventional immunoassays for blood sampling for cardiac troponin measurement, and identified a protocol based on 0–3 h assessment when using HS techniques, or 0–3–6 h when conventional (i.e., contemporary-sensitive) methods are used (32).
Conclusions
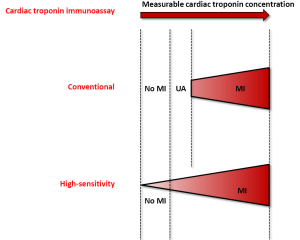
The rather short history (i.e., approximately 15 years) of diagnostic algorithms for serial cardiac troponin testing has led to a constantly evolving and unquestionably chaotic scenario (Table 1). However, recent evidence attests that the rationale use HS cardiac troponin immunoassays may contribute to disperse the overwhelming fog, provided that reasonable criteria are defined (35). The major breakthrough, strictly following the development of HS techniques for measuring cardiac troponins, has been represented by the global redefinition of myocardial ischemic injury, with the virtual disappearance of unstable angina. Many previous diagnoses of unstable angina have been in fact reclassified as “real” MIs, wherein the improved analytical sensitivity of HS immunoassays have allowed to identify low but clinically significant amounts of cardiac troponins in serum or plasma, which would ultimately mirror the presence of irreversible cardiac injury (Figure 1).
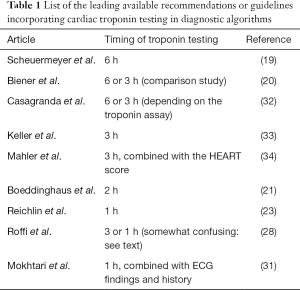
Full table
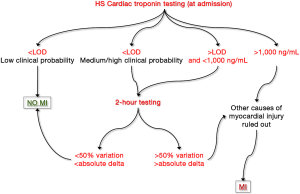
A “common sense” algorithm, including the many protocols that have been proposed so far, can hence be developed and hopefully validated in clinical studies (Figure 2). Briefly, a HS cardiac troponin value at presentation lower than the limit of detection (LOD) of the assay in a patient with low clinical probability of MI would enable safe rule out of ACS, with risk of missing an acute ischemic event or experimenting MACEs lower than 0.1–0.3% (36). Conversely, a cardiac troponin value lower <LOD in a patient with medium/high clinical probability of MI, as well as a value comprised between the LOD and 1,000 ng/mL (whatever the clinical probability) both necessitate additional testing. If we would all agree that 3-h sampling is probably unnecessary for safe rule out of MI using HS techniques, many reasons also suggest that 1 h testing is practically and clinically questionable. A much better compromise seems hence represented by 2-h testing, a time window that would allow to obtaining clinically valuable information, but that is also affordable in the vast majority of healthcare facilities. A significant variation of cardiac troponin value at 2 h (i.e., >50% or greater than absolute delta specific for each immunoassay) would hence suggest that a major myocardial damage is ongoing, whereas a variation lower than these thresholds is compatible with the presence of a chronic myocardial injury. Finally, very high cardiac troponin values (e.g., those exceeding 1,000 ng/mL) do not require additional time points for achieving a final diagnosis of severe myocardial injury.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Goodacre S, Cross E, Arnold J, et al. The health care burden of acute chest pain. Heart 2005;91:229-30. [Crossref] [PubMed]
- Conti A, Paladini B, Toccafondi S, et al. Effectiveness of a multidisciplinary chest pain unit for the assessment of coronary syndromes and risk stratification in the Florence area. Am Heart J 2002;144:630-5. [Crossref] [PubMed]
- Cervellin G, Rastelli G. The clinics of acute coronary syndrome. Ann Transl Med 2016;4:191.
- Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome?: the rational clinical examination systematic review. JAMA 2015;314:1955-65. [Crossref] [PubMed]
- Rijnbeek PR, van Herpen G, Bots ML, et al. Normal values of the electrocardiogram for ages 16-90 years. J Electrocardiol 2014;47:914-21. [Crossref] [PubMed]
- Body R, Cook G, Burrows G, et al. Can emergency physicians 'rule in' and 'rule out' acute myocardial infarction with clinical judgement? Emerg Med J 2014;31:872-6. [Crossref] [PubMed]
- Chandra A, Lindsell CJ, Limkakeng A, et al. Emergency physician high pretest probability for acute coronary syndrome correlates with adverse cardiovascular outcomes. Acad Emerg Med 2009;16:740-8. [Crossref] [PubMed]
- Cervellin G, Lippi G. Of MIs and men--a historical perspective on the diagnostics of acute myocardial infarction. Semin Thromb Hemost 2014;40:535-43. [Crossref] [PubMed]
- World Health Organization. Myocardial infarction community registers. In: Public Health in Europe, Paper No. 5, Regional Office for Europe. Copenhagen, Denmark: 1976; WHO.
- Wagner GS, Roe CR, Limbird LE, et al. The importance of identification of the myocardial-specific isoenzyme of creatine phosphokinase (MB form) in the diagnosis of acute myocardial infarction. Circulation 1973;47:263-9. [Crossref] [PubMed]
- Silva DP Jr, Landt Y, Porter SE, et al. Development and application of monoclonal antibodies to human cardiac myoglobin in a rapid fluorescence immunoassay. Clin Chem 1991;37:1356-64. [PubMed]
- Hamm CW, Goldmann BU, Heeschen C, et al. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med 1997;337:1648-53. [Crossref] [PubMed]
- Alpert JS, Thygesen K, Antman E, et al. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959-69. [Crossref] [PubMed]
- Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Eur Heart J 2007;28:2525-38. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020-35. [Crossref] [PubMed]
- Lippi G, Montagnana M, Aloe R, et al. Highly sensitive troponin immunoassays: navigating between the scylla and charybdis. Adv Clin Chem 2012;58:1-29. [Crossref] [PubMed]
- Lippi G. Biomarkers: novel troponin immunoassay for early ACS rule-out. Nat Rev Cardiol 2016;13:9-10. [Crossref] [PubMed]
- Than M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol 2012;59:2091-8. [Crossref] [PubMed]
- Scheuermeyer FX, Innes G, Grafstein E, et al. Safety and efficiency of a chest pain diagnostic algorithm with selective outpatient stress testing for emergency department patients with potential ischemic chest pain. Ann Emerg Med 2012;59:256-64.e3. [Crossref] [PubMed]
- Biener M, Mueller M, Vafaie M, et al. Comparison of a 3-hour versus a 6-hour sampling-protocol using high-sensitivity cardiac troponin T for rule-out and rule-in of non-STEMI in an unselected emergency department population. Int J Cardiol 2013;167:1134-40. [Crossref] [PubMed]
- Boeddinghaus J, Reichlin T, Cullen L, et al. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction by use of high-sensitivity cardiac troponin I. Clin Chem 2016;62:494-504. [Crossref] [PubMed]
- Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012;172:1211-8. [Crossref] [PubMed]
- Reichlin T, Twerenbold R, Wildi K, et al. Prospective validation of a 1-hour algorithm to rule-out and rule-in acute myocardial infarction using a high-sensitivity cardiac troponin T assay. CMAJ 2015;187:E243-52. [Crossref] [PubMed]
- Apple FS, Jesse RL, Newby LK, et al. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical issues for biochemical markers of acute coronary syndromes. Circulation 2007;115:e352-5. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C. Biological samples transportation by drones: ready for prime time? Ann Transl Med 2016;4:92. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C, Cervellin G. Point of care troponin testing: rules and regulations. J Electrocardiol 2013;46:727-8. [Crossref] [PubMed]
- Lippi G, Cervellin G. Letter by Lippi and Cervellin regarding article, "High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease Circulation 2013;127:e353. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med 1980;302:1109-17. [Crossref] [PubMed]
- Than M, Herbert M, Flaws D, et al. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the Emergency Department?: a clinical survey. Int J Cardiol 2013;166:752-4. [Crossref] [PubMed]
- Mokhtari A, Borna C, Gilje P, et al. A 1-h combination algorithm allows fast rule-out and rule-in of major adverse cardiac events. J Am Coll Cardiol 2016;67:1531-40. [Crossref] [PubMed]
- Casagranda I, Cavazza M, Clerico A, et al. Proposal for the use in emergency departments of cardiac troponins measured with the latest generation methods in patients with suspected acute coronary syndrome without persistent ST-segment elevation. Clin Chem Lab Med 2013;51:1727-37. [Crossref] [PubMed]
- Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011;306:2684-93. [Crossref] [PubMed]
- Mahler SA, Miller CD, Hollander JE, et al. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol 2013;168:795-802. [Crossref] [PubMed]
- Lippi G, Cervellin G. Cardiospecific troponin immunoassays: How low is it worth to go? Eur J Intern Med 2016;30:e7-8. [Crossref] [PubMed]
- Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015;386:2481-8. [Crossref] [PubMed]


