2016 reflections on the favorable cost-benefit of lung cancer screening
Rational economic behavior theory suggests that those trying to maximize healthcare value should favor interventions that cost less and have the same or better outcomes than the alternatives. The weak economy has increased interest in cost-benefit analysis as well as interest in comparing alternatives, often called cost-effectiveness analysis. Spending on healthcare is revenue to hospitals, physicians, pharmaceutical companies and others. For this spending, what benefits or outputs accrue to the patient, to the employer, to the payer or to society? In the world of cost-effectiveness, the option that costs less for similar outputs is better. But advocates, practitioners, policymakers and patients may wonder about conflicting cost-effectiveness results for lung cancer screening (and other interventions), with one study reporting screening costs under $30,000 (1) per quality-adjusted life year gained, which would meet most criteria for good cost-effectiveness, but another study estimating the costs at more than $110,000 (2).
As with other areas of study, details matter in cost-effectiveness studies. In the authors’ experience, large differences in studies’ outcomes generally reflect large differences in basic assumptions. For example, the biggest difference in the two studies mentioned in the previous paragraph was that the $30,000 figure is based on the assumption that lung cancer screening was about as effective in shifting diagnosed cancers to early, curable stages as in the National Lung Screening Trial (NLST) (3), while for the $110,000 figure, lung cancer screening had zero impact on stage at diagnosis or mortality, and the only benefit was from an accompanying smoking cessation program.
Screening often receives higher scrutiny than narrowly-focused interventions, in part because screening can affect millions of lives. For example, the population eligible for lung cancer screening according to NLST criteria is about 8.6 million based on the 2010 census (4). Annual screening at $200 per screening would cost $1.72 billion if all eligible people were screened. Contrast this to a rare disease such as amyotrophic lateral sclerosis (ALS), with about 6,400 patients diagnosed each year (5). A hypothetical $100,000 one-time treatment for ALS would cost $640 million per year, but would affect far fewer patients. While cost-effectiveness analysis is used in comparing different ways to spend the healthcare dollars, issues such as scale, societal benefits, and practicality must also be considered (6).
The growing importance of cost-benefit studies
US spending on healthcare has increased from 13.3% of gross domestic product (GDP) in 1995 to 17.5% in 2014 (7); many other countries are likewise struggling with their health spending. Healthcare spending growth has, in effect, squeezed income and spending on other services such as education. Ultimately, payers can simply pay less or otherwise constrain spending. To promote a possibly more palatable alternative to crude cost cutting, interested organizations (for- and non-profit) employ economists and others to create arguments supporting increased spending on their products or services. Governments, advocacy, industry, and lobbying organizations, and medical supply and pharmaceutical companies all hire economists and other researchers to support their positions. Most research examines incremental changes in the context resulting of the status quo of healthcare financing and structure and, therefore, could be perceived as supporting the status quo. Studies that consider broad system change are ambitious and rare (8).
The literature that has emerged assigns value to an amazing array of benefits that could accrue from spending on a particular intervention, whether that intervention is a drug, service, device, surgery or even research; the benefits considered often extend beyond improvements in health. These benefits include promises of reduced spending on some other healthcare services and better productivity by employees with a particular condition (or whose spouse or dependent has a particular condition). Most research focuses on therapies that represent additional spending; relatively few address inefficiencies in the status quo, even though widely cited estimates from diverse organizations characterize about 30% of US healthcare spending as waste. This is puzzling because the potential financial gain from waste dwarfs the gains innovative therapies claim.
As healthcare spending has grown faster than GDP, the amount of research into the value of healthcare interventions has also grown. Various terms are used for the study of cost and value, including cost-benefit (assigning a value to an outcome), cost-effectiveness (comparing the relative benefits of two or more options), and incremental cost effectiveness (a variation of cost effectiveness). As academic research into the health economics has grown, nomenclatures and specialties were invented. For example, the term “quality adjusted life years” (QALYs) attempts to include perceived patient values on their preferences for quality of life in addition to simple survival and was developed in the 1970s (9). Using QALYs, we might compare a ventilator treatment that extends a person’s life by 6 months and costs $250,000 to a treatment that extends a person’s life by 2 months, but in a wheelchair, and costs $100,000; the limits of the usefulness of such comparisons has been noted (10). The term “pharmacoeconomics” was apparently first publicly used in 1987 by an employee of Upjohn, now part of Pfizer (11); today pharmacoeconomics courses and degrees are offered at dozens of colleges (12), and the field has a professional society and journal (13). The costs of absenteeism due to illness or accident have been financed for decades through disability insurance, but “presenteeism” is a more recent introduction in the years around 2000 (14) and refers to reduced productivity by workers who are present on the job.
Despite the increasing concern over value in healthcare, cost-benefit studies are generally not easily incorporated into insurance decisions. The US insurance industry, including the federal Medicare program, uses actuaries and not economists to set rates and other financial items, and companies are required by regulators to have sign-offs by actuaries. Cost-benefit studies are often performed by economists. Actuaries typically work in an environment where cost data is audited. In health insurance, forecasts (especially those used to develop premium rates) will be compared to the actual results that will appear in audited financial statements; the data used for analysis typically comes from sources that balance to audited financial statements. Economists may examine the relationship of supply and demand and how large- or small-scale forces affect consumption of particular items. To oversimplify, an economist may forecast how public health interventions like vaccination will affect the nation’s prosperity or a society’s willingness to pay for the vaccination, while an actuary will forecast how much a payer may spend on such vaccinations. Understanding the different approaches of actuaries and economists can help explain why payers may not be influenced by seemingly strong cost-benefit analyses, and to recognize potential pitfalls in such analyses.
According to the US Bureau of Labor Statistics, in 2014 the pharmaceutical manufacturing industry employed about 90 economists and no actuaries, while the health insurance industry employed about 50 economists and over 2000 actuaries (15).
Cost-benefit study unorthodoxy: differences with clinical trials in goals and methods
Most cost-benefit studies of emerging technologies are projections based on models as opposed to results of prospectively designed trials. Academic researchers who believe randomized controlled trials are the gold standard of evidence may tend to disregard such projections. However, such models are generally the best or only tools available to individuals who must make decisions about how to allocate limited resources.
An alternative to modeling might seem to be the tabulation of clinical trial costs. The actual costs incurred in clinical trials are problematic for the purpose of modeling population impacts. Trial costs reflect the institutions participating in the trial, which may be academic institutions and not community-based providers. Some of the procedures or drugs provided to patients because the patient is in a clinical trial may not be appropriate or necessary or practical if the care were rendered outside the trial. There may be duplication of trial services with the patient’s routine, non-trial care; alternatively, services not obtained through the trial may not be captured in the patient record. Furthermore, payment mechanisms and the collection of data for “payable services” in a clinical trials are likely different from prevailing payment systems. So, even if costs are comprehensively tabulated during the trial period, significant adjustments would be required. So, even if clinical trial costs are tabulated, the resulting cost-benefit study will be very much about modeling.
There are many technical approaches to modeling cost-benefit, and particular studies may combine different methods, almost as an assembly line may combine parts from different manufacturers. Methods include stochastic simulations and probabilistic/deterministic approaches. These methods typically implement the results of decision trees that capture important outcomes, which, in turn, may have been developed from clinical trials or observational studies. Generally, studies begin with a target population (for example, smokers and ex-smokers) which is a segment of a larger national population.
The goal of most cost-benefit studies is to determine answers for a population, but it is common to get there by simulating individuals from this population (perhaps defined by their age, sex, smoking history or other characteristics). These individuals will be treated (virtually, of course) according to the rules of the decision trees chosen by the modeler. For many models, one decision tree will reflect the status quo of treatment (e.g., no lung cancer screening), while other decision trees will have the same patients go through the steps of the intervention—and will reflect expected results of the intervention [e.g., the process of low dose computed tomography (LDCT) screening and follow-up]. Often the intervention “arm” tests various scenarios (e.g., different ages for the first annual LDCT screening).
Modeling is very different from analyzing the results of clinical trials. Those accustomed to analyzing clinical trial results may be puzzled that modelers combine data from disparate sources as a basic premise of randomized controlled trials is to eliminate or randomize outside influences. For example, the age-sex distribution of smokers and ex-smokers may come from a government survey [e.g., the National Health and Nutrition Examination Survey (NHANES)], while the status quo incidence and survival of lung cancer by stage, age, and sex may come from registry data [e.g., Surveillance, Epidemiology and End Result (SEER)], the costs of treating cancer from yet another source (e.g., the Medicare 5% limited dataset), and the stage-shift created by screening from yet another source [e.g., the International Early Lung Cancer Action Program (IELCAP)]. Needless to say, much thinking and care is needed when combining data from multiple sources. Non-practitioners may be comforted by knowing that such melding is common in actuarial work: it was how actuaries first developed survival curve methodologies 100 years before Kaplan-Meier’s famous 1957 paper (16), and the use of multiple sources is noted in Actuarial Standards of Practice (17).
Basics of cost-benefit analysis
Cost-benefit analysis is typically measured as “cost per life-year saved” or “cost per QALY saved”. These may be presented as “$30,000 per life-year saved”, or “$30,000 per QALY saved”. The numerator is the cost, and the denominator is usually an outcome. Costs and outcomes are separate metrics but must be defined consistently, and their definitions must vary depending on the situation (and the intended audience). This section examines key choices in the definition of cost and outcome metrics.
Table 1 shows key considerations and illustrates how these can vary under different circumstances. Note that the choices of cost and outcome can be highly “correlated”: a correctly performed payer-perspective analysis will measure outcomes relevant to payers, and will in turn require the use of a cost basis appropriate to payers.

Full table
The incremental cost-effectiveness ratio (ICER) is a derivative concept, where two interventions are compared by examining the difference in costs and the difference in outcomes.
The favorable cost-benefit of lung cancer screening
Insurance programs in the US use monthly cost per covered life to manage many aspects of their business. Metrics developed this way are termed, per-member-per-month (PMPM). Estimating the cost of services is, of course, a simpler exercise than estimating cost-benefit, but even here different assumptions lead to different results. Roth et al. (21) estimated costs to Medicare of screening at $2.22 PMPM, more than double the $1.02 PMPM developed by Pyenson. Both estimates are relatively modest compared to the Medicare $672 PMPM expenditure for 2012 part A and part B benefits. The most significant differences in the two studies are that Roth assumes a somewhat higher average fee for LDCT than Medicare’s reimbursement plus additional physician evaluation or counseling services associated with the screening process. Despite differences in assumptions, both estimates support the low cost of lung cancer screening.
Cost-benefit studies of lung cancer screening fall into two categories based on whether they assume screening is effective in increasing the portion of early stage cancers detected—the stage-shift. NLST demonstrated this clinical effectiveness in 2011 through a randomized controlled trial. However, the stage shift demonstrated over the prior decade in Claudia Henschke’s I-ELCAP observational studies proved to be consistent with the later NLST stage-shift results. Modeling based on either NLST or Henschke are the major sources for recent studies.
Table 2 compares key assumptions for recent studies on the cost-effectiveness of LDCT screening.
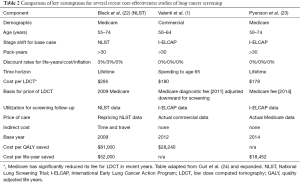
Full table
Discussion
In retrospect, evidence for the effectiveness and cost-effectiveness of lung cancer screening for high-risk populations should have been considered conclusive long before NLST results were published. To the authors, the key evidence was as follows:
- Most lung cancer is concentrated in a group of identifiable high-risk patients—smokers, ex-smokers, and certain environmental/occupational exposures. Screening does not need to be done for everyone. This contrasts to colorectal and breast cancers where screening extends to the entire population based on age. In contrast to the 8.6 million eligible for LC screening, the FDA reports about 39 million actual mammograms annually (not all of which are screening) (25);
- Treated, early stage lung cancers have dramatically better survival than late-stage lung cancers (26). Stage IIIB non-small cell lung cancer (NSCLC) in the under-65 population has over six times the mortality of treated stage 1A. The corresponding figure for stage IV NSCLC is more than ten time that of treated stage 1A;
- I-ELCAP’s long observational series demonstrates that LDCT screening dramatically shifts diagnosed lung cancers to earlier stages (27);
- LDCT screening is low-cost. Medicare reimbursement was less than $200 per LDCT in 2014 (23).
The combination of concentrated risk, large mortality differences, demonstrated early detection ability, and low cost all point to large opportunities. This explains why researchers who incorporate the above elements develop similar, favorable results—despite some factual errors and methodological disputes.
Detailed cost-benefit analyses have provided some interesting findings. The medical costs in the US of treating patients with early stage or late stage lung cancers did not differ by as much as we would have hoped. This is perhaps an artifact of US reimbursement. In other countries, the less intense treatments for early stage cancers may be much lower than for later stage cancer. The fact that survivors of early stage lung cancers live extra years and incur other medical costs means that saving lives incurs extra costs. We also found that even large variations in how patients are followed after the annual screening or after the finding of a suspicious nodule have little impact on cost-effectiveness. That’s because very few patients require any subsequent work-up, so the cost of the LDCT dominates screening cost.
There are several lessons of the effectiveness and cost-effectiveness of lung cancer screening for public health and healthcare spending:
(I) Scale matters. High-frequency, concentrated risks, high mortality, and high costs mean the topic is important;
(II) Effectiveness is critical. Many medical cost efforts are focused on “high cost patients”, for obvious reasons (28). However, the ability to affect future costs after the patient has begun to incur high costs has proved elusive, as has the ability to predict at-risk patients and to intervene to alter their course (29);
(III) Real-world healthcare financing is important. Real-world data can be ugly—it may be full of administrative errors, inappropriate treatments, extraneous influences, and various biases. However, when it comes to payers’ audited financial statements, real-world data is what is available to them. Cost effectiveness studies will be more credible to payers to the extent that assumptions are based on real-world data.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Villanti AC, Jiang Y, Abrams DB, et al. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One 2013;8:e71379. [Crossref] [PubMed]
- McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol 2011;6:1841-8. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Ma J, Ward EM, Smith R, et al. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer 2013;119:1381-5. [Crossref] [PubMed]
- Facts You Should Know. Available online: http://www.alsa.org/about-als/facts-you-should-know.html
- The Cancer Drugs Fund. Available online: https://www.england.nhs.uk/ourwork/cancer/cdf/
- National Health Expenditure Data. Available online: . Accessed Jan 31, 2016https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html?redirect=/NationalHealthExpendData/
- Parston G, McQueen J, Patel H, et al. The Science And Art Of Delivery: Accelerating The Diffusion Of Health Care Innovation. Health Aff (Millwood) 2015;34:2160-6. [Crossref] [PubMed]
- Zeckhauser R, Shepard D. Where Now for Saving Lives? Law Contemp Probl 1976;40:5-45. [Crossref]
- Kind P, Lafata JE, Matuszewski K, et al. The use of QALYs in clinical and patient decision-making: issues and prospects. Value Health 2009;12 Suppl 1:S27-30. [Crossref] [PubMed]
- Mauskopf JA. Why study pharmacoeconomics? Expert Rev Pharmacoecon Outcomes Res 2001;1:1-3. [Crossref] [PubMed]
- Compare Colleges & Universities. Available online: http://colleges.startclass.com/
- Value in Health. Available online: http://www.ispor.org/
- Johns G. Presenteeism in the workplace: A review and research agenda. J Organiz Behav 2010;31:519-42. [Crossref]
- Occupational Employment Statistics Query System. Available online: . Accessed Dec 27, 2015.http://data.bls.gov/oes/
- Gail MH, Benichou J. Encyclopedia of Epidemiologic Methods. P892. Available online: https://books.google.com/books?id=8qIMMbsO784C&pg=PA892&lpg=PA892&dq=history+of+actuarial+tables+Kaplan-Meier&source=bl&ots=Llb9UrTRt_&sig=YYVFpia6EWa3WliEv35zxAddsk0&hl=en&sa=X&ved=0ahUKEwjQuL-Q6_7JAhVMdj4KHfkfCpUQ6AEIUjAJ#v=onepage&q=history%20of%20actuarial%20tables%20Kaplan-Meier&f=false
- Actuarial Standard of Practice 23, Data Quality. Actuarial Standards Board. Available online: http://www.actuarialstandardsboard.org/standards-of-practice/
- Bach P. Don’t believe the hype about lung-cancer screenings. Slate Nov 15, 2010. Available online: http://www.slate.com/articles/health_and_science/medical_examiner/2010/11/ct_scam.single.html#pagebreak_anchor_2
- Pyenson B, Pickhardt PJ, Sawhney TG, et al. Medicare cost of colorectal cancer screening: CT colonography vs. optical colonoscopy. Abdom Imaging 2015;40:2966-76. [Crossref] [PubMed]
- Baker R, Bateman I, Donaldson C, et al. Weighting and valuing quality-adjusted life-years using stated preference methods: preliminary results from the Social Value of a QALY Project. Health Technol Assess 2010;14:1-162. [Crossref] [PubMed]
- Roth JA, Sullivan SD, Goulart BH, et al. Projected Clinical, Resource Use, and Fiscal Impacts of Implementing Low-Dose Computed Tomography Lung Cancer Screening in Medicare. J Oncol Pract 2015;11:267-72. [Crossref] [PubMed]
- Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med 2014;371:1793-802. [Crossref] [PubMed]
- Pyenson BS, Henschke CI, Yankelevitz DF, et al. Offering lung cancer screening to high-risk medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits 2014;7:272-82. [PubMed]
- Curl PK, Kahn JG, Ordovas KG, et al. Understanding Cost-Effectiveness Analyses: An Explanation Using Three Different Analyses of Lung Cancer Screening. AJR Am J Roentgenol 2015;205:344-7. [Crossref] [PubMed]
- MQSA National Statistics. Available online: http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/FacilityScorecard/ucm113858.htm
- Goldberg SW, Mulshine JL, Hagstrom D, et al. An actuarial approach to comparing early stage and late stage lung cancer mortality and survival. Popul Health Manag 2010;13:33-46. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators, Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- Congressional Budget Office. High Cost Beneficiaries. May 2015. Available online: https://www.cbo.gov/publication/16487
- Urato C, McCall N, Cromwell J, et al. Evaluation of the extended Medicare Care Management for High Cost Beneficiaries (CMHCB) demonstration: Health Buddy® program at Montefiore. Final report: Prepared for Centers for Medicare & Medicaid Services, Baltimore, MD. October 2013. Research Triangle Park, NC: RTI International. Available online: http://www.rti.org/pubs/cmhcb-healthbuddymontefiore.pdf


