Laparoscopic Finney pyloroplasty in the emergency setting: first case report in the literature and technical challenges
Introduction
Several laparoscopic techniques are currently available to treat the emergent complications of peptic ulcer disease (PUD), which include gastrointestinal perforation, hemorrhage and obstruction. Many studies have demonstrated the feasibility and the advantages of laparoscopic surgery (LS) compared to the traditional open surgical approach in the emergency setting (1). We report the first case in the literature, to the best of our knowledge, of a laparoscopic Finney pyloroplasty (LFP) in order to address the challenges when performing a laparoscopic pyloroplasty procedure in a case of perforated DU with gastric outlet obstruction (GOO) and significant loss of mural substance in which direct ulcer repair is impossible.
Case presentation
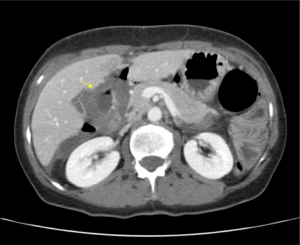
A 50-year-old woman was referred to the Emergency Department (ED) of an Italian General Hospital with sudden-onset acute abdominal pain accompanied by nausea and unintentional weight loss of 5 kg over 3 weeks. The patient did not take non-steroidal anti-inflammatory drugs (NSAIDs), smoke, or drink alcohol, and her history did not include any conditions predisposing to peptic ulceration. Physical examination revealed guarding and rebound tenderness in the hypochondria and epigastrium. Laboratory investigations yielded a white cell count of 21,700×103/µL alongside normal hemoglobin and electrolyte levels. On abdominal radiography there was free subdiaphragmatic gas, meteoric hyperinflation of the entire colon and widespread fecal residue with multiple air-fluid levels. An abdominopelvic contrast-enhanced computed tomography (CT) confirmed massive pneumoperitoneum, with free gas confined within the intra-hepatic fissure and periportal free gas tracking posterior to the portal vein, a fluid-filled distended stomach, as well as a periduodenal fluid collection with mural thickening (Figure 1). A preoperative diagnosis of generalized peritonitis and DU perforation was established. After aggressive fluid resuscitation and preoperative optimization, the patient was transferred to the operating theatre, induced by general anesthesia and positioned supine, with legs straight and abducted. The primary surgeon was positioned between the patient’s legs, with the assistant to the patient’s right. A first dose of antibiotics (1.2 g of intravenous co-amoxiclav) was administered on induction. Following skin preparation and draping, pneumoperitoneum was established with the open Hasson technique, 4 trocars (ENDOPATH® XCEL dilating-tip trocar, Ethicon Endo-Surgery, Guaynabo, Puerto Rico 00969 USA) were inserted and views were established with a 30° camera (Figure 2A).
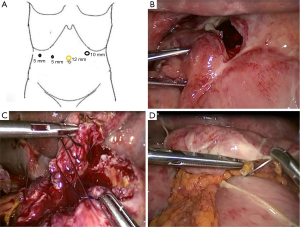
The first operative step consisted of abdominal cavity exploration, confirming the diagnosis of generalized peritonitis and retracting the liver, which revealed a 25 mm perforated DU in the first and second part of the duodenum (D1–D2), with a deforming retraction scar (Figure 2B). Although the liver was retracted via the left hypochondrial port, direct ulcer repair could not be achieved, necessitating a Kocher maneuver. The second operative step therefore included adhesiolysis and duodenal mobilization from the retraction scar and related fibrous tissue. The extensive Kocher maneuver performed also served to reduce tension on the subsequent anastomotic suture. The duodenum was dissected from proximal attachments to the gastrohepatic ligament in order to allow approximation of D2 to the distal antral greater curvature. The ulcer borders were excised and extracted with an ENDOPOUCH® (Ethicon) specimen retrieval bag and sent for histopathological analysis. Microscopy of the 50×30×20 mm3 tissue section revealed inflammatory cells and granulation tissue, typical of peptic ulceration with exclusion of any malignancy.
Following excision of the inferior and superior rims of the ulcer and exposure of D2, we performed a longitudinal duodenotomy and a longitudinal gastrostomy, as per the FP.
A transmural incision was made along the inverted U-shaped line running from the gastric antrum 4 to 5 cm proximal to the pylorus curving through the duodenal bulb and down to D2. Duodenal mobilisation and the longitudinal gastrostomy and pylorotomy were performed with the Ethicon ULTRACISION® Harmonic Scalpel.
Approximation of the two incisions confirmed a tension free anastomosis. Subsequently, a double-layer side-to-side anastomosis between the distal stomach and proximal duodenum was fashioned with a 3–0 violet monofilament polydioxanone suture (PDS™, Ethicon, Ethi-Endo-Clip-Suture, Lahodny, Johnson & Johnson Medical S.P.A. Via del Mare, 56.00040 Pomezia, Roma; Figure 2C). The posterior outer layer was formed with a continuous seromuscular suture from the apex of the inverted U-shaped incision, followed by a continuous full-thickness suture for the posterior inner layer. The anterior inner and outer layers were formed with a full-thickness interrupted absorbable suture. Omentoplasty, using the greater omentum, was then performed to consolidate the anastomosis (Figure 2D).
Finally, the abdomen was generously irrigated with 5 L of 0.9% saline solution. A nasogastric tube (NGT) was inserted, as well as two intra-abdominal drains to the paraduodenal and suprahepatic areas. The operation described above was carried out uneventfully and completely laparoscopically in 240 minutes, with an estimated blood loss of 150 mL and without blood product transfusion.
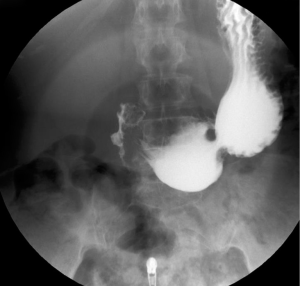
The patient was postoperatively transferred to the Intensive Care Unit and was initiated on a six-day course of intravenous metronidazole and ertapenem, according to advice from the local Microbiology Department. Endotracheal extubation took place on the 1st postoperative day with the patient hemodynamically and clinically stable. The abdominal drains were removed on the 2nd postoperative day. On the 5th postoperative day a Gastrografin® (diatrizoate meglumine and diatrizoate sodium solution) enhanced dynamic radiological study was performed, which excluded gastrointestinal leakage. The NGT was therefore removed and free fluids per os were commenced, followed by escalation to a soft diet. The patient was discharged on the 8th postoperative day with normal bloods values (full blood count, urea, electrolytes, C-reactive protein) and with a prescription for regular oral proton pump inhibitors. The repeat dynamic study at 1 month yielded normal result (Figure 3), and at her 6-month follow-up the patient remained well and asymptomatic.
Discussion
Zelickson et al. reported that PUD affects 4 million people worldwide (2-4). The number of patients requiring elective surgery for PUD has declined markedly since the widespread introduction of effective antiulcer drugs (5). PUD-related deaths arise primarily from hemorrhage and perforation, both of which can be managed with minimally invasive surgery (MIS). The evidence-based guidelines produced by the European Association for Endoscopic Surgery in 2006 and many others studies have demonstrated the superiority of laparoscopic surgery, even in the emergency setting (6,7). Furthermore, routine use of laparoscopy in the diagnosis and management of the acute abdomen has been widely accepted (8). MIS confers many advantages when compared to open surgery. Specifically, LS guarantees perfect exposure of the abdominal cavity, achieves a lower incidence of adhesion formation and wound complications, less postoperative pain and superior cosmesis. LS additionally permits earlier recovery of gastrointestinal motility with rapid resumption of daily activities (1,8). Various laparoscopic approaches and many modified techniques have been evaluated to address gastric and DU. Gastric and duodenal perforations (up to 2 cm in diameter) can be successfully treated with the use of a single-layer suture “Graham patch technique”, employing gelatin sponge or fibrin glue (9-12). Despite the variety of available methods, repair of severe duodenal wall defects which are associated with significant morbidity and mortality remains a challenge (13,14).
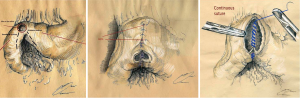
John Miller Turpin Finney [1863-1942] pioneered the pyloroplasty to alleviate GOO, as a complication of PUD (15). The original technique was a double-layer side-to-side anastomosis between the distal stomach and proximal duodenum. In the classical FP the second part of the duodenum (D2) and the distal part stomach were first approximated by means of a seromuscular suture in order to close the angle of the pylorus. Afterwards, an inverted U-shaped incision was made through D2 across the pyloric sphincter and down the pyloric canal (16). In our technique, we have respected the principles of Finney’s procedure, as illustrated in Figure 4. The posterior walls of the gastric and the duodenum portions were closed with a continuous seromuscular suture running through the inferior gastroduodenal angle to proceed onto the anterior wall of the gastroduodenostomy. Recent studies reported the feasibility of LFP in a canine model, which strongly suggests applicability to human gastrointestinal surgery since canine and human anatomy in this region is similar (17). Sánchez-Margallo et al. evaluated the technical feasibility of LFP in six dogs and successfully completed all the operations laparoscopically and postoperative studies assessed the correct function of the anastomosis (18).
This case report describes a duodenal perforation with extensive loss of mural substance and a significant retraction scar, which rendered direct ulcer repair infeasible thus necessitating FP. This procedure also allowed removal of the ulcer for histological examination, which confirmed the intraoperative diagnosis. Nevertheless, there are certain important considerations to undertaking a LFP. Firstly, the size of duodenal ulceration must be evaluated. In fact, a direct suture is unmanageable in full thickness peptic ulcers of at least 2 cm diameter, which are related to a retraction scar. Secondly, the site of perforation determines the most appropriate operation. A posterior perforation, or one, which involves an extensive area of D2, may necessitate partial gastrectomy. Thirdly, the surgeon’s experience must be considered as the FP may actually cause GOO via local scarring or edema, which then requires NGT decompression and conservative treatment. Additionally, due to severe adhesions which may form between the gallbladder and duodenum, intraoperative cholangiography and cholecystectomy may be necessary to evaluate the biliary anatomy and avoid biliary injuries, particularly in the setting of a bleeding perforated DU which requires suturing (19). In this particular emergency case however, we decided against cholangiography and cholecystectomy since the anatomy of the external biliary tree was clearly visualized without variation, and there was no bleeding.
Local case volume in LS, as well as operator experience allowed for accurate intraoperative diagnosis, as well as completion of the procedure totally laparoscopically. Review of the medical literature does not reveal any reports of LFP in the emergency setting. This procedure, with an open approach, is in fact undertaken in cases of benign GOO, and is only rarely performed in the emergency setting since it is more technically challenging than the Heineke-Mikulicz pyloroplasty (20-23). FP can be used particularly when the site of the perforation is too wide and located in D2, to ensure good caliber of the pylorus when no healthy tissue around the ulcer can provide good closure of the duodenal defect (18,24). As this is a single case report, further studies are required to define the long-term outcomes and/or compare LS to open surgery in perforated DU with GOO. In conclusion, we report the first case of LFP to treat a perforated DU with GOO in the emergency setting, and believe this is both an effective and feasible approach with a long learning curve.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Sauerland S, Agresta F, Bergamaschi R, et al. Laparoscopy for abdominal emergencies: evidence-based guidelines of the European Association for Endoscopic Surgery. Surg Endosc 2006;20:14-29. [Crossref] [PubMed]
- Clancy TE, Asheley SW. Procedures for benign and malignant gastric and duodenal disease. ACS Surgery: Principles and Practice. Available online: http://www.slideshare.net/medbookonline/acs0520-procedures-for-benign-and-malignant-gastric-and-duodenal-disease-2006
- Thorsen K, Søreide JA, Kvaløy JT, et al. Epidemiology of perforated peptic ulcer: age- and gender-adjusted analysis of incidence and mortality. World J Gastroenterol 2013;19:347-54. [Crossref] [PubMed]
- Zelickson MS, Bronder CM, Johnson BL, et al. Helicobacter pylori is not the predominant etiology for peptic ulcers requiring operation. Am Surg 2011;77:1054-60. [PubMed]
- Chung SC, Li AK. Helicobacter pylori and peptic ulcer surgery. Br J Surg 1997;84:1489-90. [Crossref] [PubMed]
- Catani M, De Milito R, Romagnoli F, et al. Laparoscopic colorectal surgery in urgent and emergent settings. Surg Laparosc Endosc Percutan Tech 2011;21:340-3. [Crossref] [PubMed]
- Guidelines for Laparoscopic Resection of Curable Colon and Rectal Cancer. Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), 07/2006. Available online: http://www.francescobiondo.it/approfondimenti/Malattie%20del%20colon-retto/Area%20Medici/Guidelines%20for%20lap%20resection%20of%20colon%20&%20rectal%20cancer.pdf
- Kirshtein B, Roy-Shapira A, Lantsberg L, et al. The use of laparoscopy in abdominal emergencies. Surg Endosc 2003;17:1118-24. [Crossref] [PubMed]
- Lam PW, Lam MC, Hui EK, et al. Laparoscopic repair of perforated duodenal ulcers: the"three-stitch" Graham patch technique. Surg Endosc 2005;19:1627-30. [Crossref] [PubMed]
- Kirshtein B, Bayme M, Mayer T, et al. Laparoscopic treatment of gastroduodenal perforations: comparison with conventional surgery. Surg Endosc 2005;19:1487-90. [Crossref] [PubMed]
- Lau H. Laparoscopic repair of perforated peptic ulcer: a meta-analysis. Surg Endosc 2004;18:1013-21. [Crossref] [PubMed]
- Cienfuegos JA, Rotellar F, Valentí V, et al. Giant duodenal ulcer perforation: a case of innovative repair with an antrum gastric patch. Rev Esp Enferm Dig 2012;104:436-9. [Crossref] [PubMed]
- Gupta S, Kaushik R, Sharma R, et al. The management of large perforations of duodenal ulcers. BMC Surg 2005;5:15. [Crossref] [PubMed]
- Sung JJ. Marshall and Warren Lecture 2009: peptic ulcer bleeding: an expedition of 20 years from 1989-2009. J Gastroenterol Hepatol 2010;25:229-33. [Crossref] [PubMed]
- Haubrich WS. Finney of the Finney pyloroplasty. Gastroenterology 2004;126:56. [Crossref] [PubMed]
- Hendry WG. The treatment of peptic ulceration by vagotomy and Finney pyloroplasty. Postgrad Med J 1961;37:137-41. [Crossref] [PubMed]
- Sánchez-Margallo FM, Ezquerra-Calvo LJ, Soria-Gálvez F, et al. Comparison of the effect of laparoscopic and conventional pyloric surgery on gastric emptying in dogs. Vet Radiol Ultrasound 2005;46:57-62. [Crossref] [PubMed]
- Sánchez-Margallo FM, Loscertales B, Díaz-Güemes I, et al. Technical feasibility of laparoscopic Finney pyloroplasty examined in a canine model. Surg Endosc 2007;21:136-9. [Crossref] [PubMed]
- Di Saverio S, Bassi M, Smerieri N, et al. Diagnosis and treatment of perforated or bleeding peptic ulcers: 2013 WSES position paper. World J Emerg Surg 2014;9:45. [Crossref] [PubMed]
- Søreide K, Sarr MG, Søreide JA. Pyloroplasty for benign gastric outlet obstruction--indications and techniques. Scand J Surg 2006;95:11-6. [PubMed]
- Schlöricke E, Hoffmann M, Zimmermann M, et al. Simultaneous laparoscopic pyloroplasty and ileocecal resection in Crohn's disease. Acta Chir Iugosl 2012;59:117-20. [Crossref] [PubMed]
- Lal P, Vindal A, Hadke NS. Controlled tube duodenostomy in the management of giant duodenal ulcer perforation: a new technique for a surgically challenging condition. Am J Surg 2009;198:319-23. [Crossref] [PubMed]
- Nussbaum MS, Schusterman MA. Management of giant duodenal ulcer. Am J Surg 1985;149:357-61. [Crossref] [PubMed]
- Sánchez-Margallo FM, Soria-Gálvez F, Ezquerra-Calvo LJ, et al. Comparison of ultrasonographic characteristics of the gastroduodenal junction during pyloroplasty performed laparoscopically or via conventional abdominal surgery in dogs. Am J Vet Res 2003;64:1099-104. [Crossref] [PubMed]


