Harnessing the immune system to improve cancer therapy
Introduction
Cancer immunotherapy involves the exploitation of the immune system’s machinery to recognize, target and destroy cancer cells. The idea of using the immune system against cancer is based, among others, on the following properties of its components; immune cells (I) provide constant surveillance, as they continuously travel throughout the body; (II) are specifically stimulated against tumors, which are by definition antigenic and often immunogenic; and (III) protect against tumor relapse, due to induction of specific and long-lasting memory. Nevertheless, tumors escape from immunosurveillance through a well described procedure termed “cancer immunoediting”. Koebel and coworkers elegantly showed that immunoediting comprises three main sequential events: elimination, equilibrium, escape, and eventually leads to cancer growth (1).
Cancer immunotherapy came of age particularly after 2000. New knowledge on the mechanisms of anti-tumor immune responses, novel technological platforms on the production of active anti-cancer compounds and innovative advances to quantify clinical responses have resulted in improved cancer immunotherapeutic protocols for patient treatment in the clinical setting. Since Coley’s first anti-cancer intervention in 1893, major landmarks comprise the 1973 discovery of dendritic cells (DCs) (2); the 1989 development of the first chimeric antigen receptors (CARs) (3); the 1991 cloning of the first tumor antigen; and the 1995 identification of the first checkpoint molecule, namely the cytotoxic T lymphocyte-associated protein 4 (CTLA-4) (4). Licensing of clinical trials in 2000 and the first results reported at that time did not boost the enthusiasm of most cancer immunologists and oncologists. However, recently accumulated data strongly indicate measurable improvement in patient outcome and, in several cases, induction of efficient and durable responses. In the next sections, we will briefly review the most popular anti-cancer immunotherapeutic protocols and suggest possible means to exploit their synergistic potential for the benefit of cancer patients.
Classification of cancer immunotherapy strategies
Cancer immunotherapy is briefly divided into two main types of interventions: passive and active (Figure 1). The classification is based on the mechanism of action of the therapeutic agent used, as well as on the status of the patient’s immune system. In general, passive immunotherapeutics are used in cancer patients with weak, unresponsive, or of low responsiveness immune systems. Passive protocols consist of ex vivo-activated cells or molecules that once found inside the body, compensate for missing or deficient immune functions. Among others, this category includes the infusion of tumor-specific antibodies, the systemic administration of recombinant cytokines and the adoptive transfer of immune cells pre-activated to lyse tumors in vivo. On the other hand, active immunotherapy strategies aim to stimulate effector functions in vivo. To apply active immunotherapeutics, the patient’s immune system should be able to respond upon challenge, get competently stimulated and mediate effector functions. The most important active protocols comprise vaccination strategies with tumor peptides or allogeneic whole cells, the use of autologous DCs as vehicles for tumor antigen delivery, and the infusion of antibodies targeting crucial checkpoints of T cell activation. Finally, although initially considered as passive intervention, the systemic immune responses induced by oncolytic viruses shifted this novel therapeutic modality to the group of active cancer immunotherapeutics.
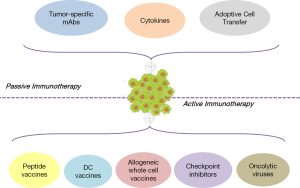
Passive cancer immunotherapy
Tumor-specific monoclonal antibodies (mAbs) target cancer-specific or cancer-associated antigens and lyse cancer cells via various mechanisms
Historically, mAbs were described as “magical bullets”. The first anti-cancer mAbs of murine origin were recognized as foreign by the patient’s immune system and the generation of human anti-mouse antibody (HAMA) responses abrogated their biological efficacy. Advances in antibody engineering resulted in greatly reducing HAMA responses and the currently used mAbs are chimeric, humanized or fully human.
The most commonly used mAbs in cancer immunotherapy are of the IgG class due to their long half-life and stability in serum. Naked anti-cancer mAbs mediate their function through directly inducing programmed cell death upon binding to tumor targets and by antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and/or antibody-dependent cellular phagocytosis (ADCP). Briefly, mAbs promote ADCC or ADCP-mediated tumor burden clearance, via interactions of their constant fragment (Fc) with Fc γ-receptors (FcγRIIIa and FcγRIIa on NK cells and macrophages, respectively) (5). For example, the chimeric mAb rituximab targets CD20 on malignant B lymphocytes facilitating recognition by immune effectors, induction of apoptosis by NK cells via perforin/granzyme release and Fas/FasL interactions, and/or phagocytosis by macrophages (6). In CDC, the activation of complement (C) factors (e.g., C1q, C3b) leads to the formation of membrane attack complexes, as well as to the recruitment of immune cells (C3a and C5a) (7). For example, it has been reported that the humanized anti-CD52 mAb alemtuzumab exerts its anti-tumor activity by solely mediating CDC in patients with chronic lymphocytic leukemia (8). The significance of both ADCC and CDC in cancer immunotherapy is evidently supported by the correlation of clinical responses to mAb therapy with polymorphisms in the FcγR and C1qA genes (6). Moreover, ADCP facilitates cross-presentation of tumor peptides derived from engulfed apoptotic cells on major histocompatibility complex (MHC) molecules and the expansion of tumor-reactive CD8+ and CD4+ T cells that, among others, prime B cells to produce host anti-tumor antibodies (Abs) (9).
Antibodies or antibody fragments can be conjugated via their Fc to radioisotopes (e.g., the anti-CD20 mAb 131I-tositumomab), cytokines [e.g., the anti-GD2/interleukin (IL)-2 fusion protein EMD 273063] and toxins (e.g., gemtuzumab ozogamicin, a fusion of a cytotoxic antibiotic to a mAb targeting CD33 on leukemic myeloblasts) (10). In Ab-directed enzyme prodrug therapy (referred to as ADEPT), an enzyme linked to the mAb Fc converts a non-toxic prodrug, given systemically, into a potent cytotoxic agent (e.g., fusion of Fc to α-lactamase that converts C-Mel into melphalan) (11). All aforementioned approaches deposit the cytotoxic agent to the vicinity of the tumor, thus minimizing adverse events.
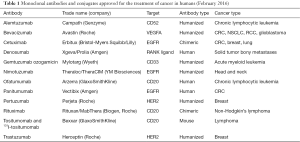
Currently, many mAbs used in cancer treatment target and bind to a certain antigen on cancer cell surface, blocking specific downstream signaling pathways and arresting cell proliferation (Table 1). Indicative examples include cetuximab and panitumumab targeting the epidermal growth factor receptor (EGFR). Both mAbs prevent binding of the activation ligand EGF and receptor dimerization, further blocking PI3K/AKT and Ras/MAPK signaling (12,13). They are used, so far, as second- and third-line treatment for metastatic colorectal cancer (CRC). Trastuzumab and pertuzumab target the truncated form of EGFR, HER2. They inhibit receptor dimerization, increase its endocytic destruction, mediate ADCC and induce apoptosis (14). Other mAbs that target the immunosuppressive tumor microenvironment also showed beneficial results in the clinical setting. Bevacizumab prevents binding of vascular endothelial growth factor (VEGF) to its receptors and inhibits angiogenesis. Its use is approved for some solid tumors (e.g., CRC), in combination with chemotherapy (15). Daclizumab, a CD25 specific mAb, efficiently depletes CD4+CD25+FoxP3+ regulatory T cells (Tregs) and is approved for treating patients with metastatic breast cancer (16). Finally, bispecific mAbs have also shown promise, acting by bridging immune effectors to cancer cells and promoting tumor cell eradication. A new class of such mAbs, the artificially produced bispecific T cell engagers (BiTEs), can induce T cell-mediated tumor elimination in the absence of T cell receptor (TCR)-MHC interactions. Blinatumomab is currently the only Food and Drug Administration (FDA) approved BiTE for treating refractory or relapsed Philadelphia chromosome-negative B-acute lymphocytic leukemia (17).
Full table
Cytokine administration demonstrates some efficacy, mainly in combinatorial anti-cancer treatments
Although being one of the first therapeutic interventions in cancer, cytokine use as monotherapy is no longer popular. The most prominent cytokines are interferon-alpha (IFN-α), IL-2 and IL-12. High dose IL-2, as well as IFN-α, received FDA approval for use in metastatic melanoma (in 1992 and 2011, respectively) and renal cell carcinoma (RCC; in 1998 and 2009, respectively), as both act pleiotropically and reportedly exert immunomodulatory effects on immune cells (18,19). IFN-α further demonstrated a marked suppressive effect on Tregs in the tumor microenvironment. Specifically, post-operative IFN-α administration in RCC patients for 4 weeks resulted in decreased frequency of tumor and peripheral blood Tregs (20). Recent in vitro data suggest that integration of IFN-α in a DC-based protocol notably improved its therapeutic efficacy (21).
IL-2 is preferably administered in combination with standard treatments, such as chemotherapy, other cytokines, peptide vaccines and mAbs. For example, the combined administration of IL-2 and IFN-α in RCC patients with lung metastases exhibited a significant survival benefit (22). In patients with advanced melanoma, administration of a gp100 peptide vaccine with IL-2 led to higher rates of clinical response, prolonged progression-free and overall survival (OS), compared to high dose IL-2 monotherapy (23).
Another widely used cytokine is IL-12, which is normally secreted from antigen presenting cells (APCs) in response to antigen stimulation. Among its other biological activities, IL-12 promotes CD4+ T cell polarization to Th1 cells, orchestrates anti-cancer responses and inhibits tumor-derived Tregs (24,25). Although the first phase II trial failed due to severe toxicity (26), IL-12 treatment of cutaneous T cell lymphoma (27), non-Hodgkin’s B cell lymphoma (28) and AIDS-associated Kaposi sarcoma (29) showed encouraging results. In addition, IL-12-based gene therapy with electroporation-mediated plasmid transfers (30) and immunocytokine approaches (e.g., NHS-IL-12) (31) have also been tested.
Adoptive cell transfer (ACT) strategies significantly improve patient outcome in solid and hematological malignancies
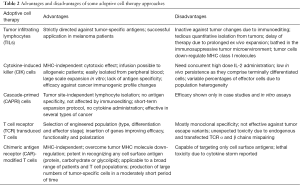
In ACT protocols, patients are treated with ex vivo expanded autologous cells, including tumor infiltrating lymphocytes (TILs), cytokine-induced killer (CIK) or cascade-primed (CAPRI) cells (Table 2). TILs are isolated by dissociation of tumor specimens into single cell suspensions and in vitro lymphocyte expansion in the presence of high dose IL-2. Promising results were shown in metastatic melanoma patients, where treatment with TILs proved highly efficient, inducing durable responses irrespective to prior therapies applied (32). Remarkably, tumor-reactive CD4+ TIL infusion in a female patient with widely spread metastatic cholangiocarcinoma resulted in regression of her liver and lung metastases (33).
CIK cells comprise a heterogeneous population, mainly consisting of CD3+CD56+ cells. They are generated upon in vitro stimulation of peripheral blood mononuclear cells with anti-CD3, IL-1β and IFN-γ, while addition of IL-2 and IL-15 further augments their effector functions (34). CIK cells are non-MHC restricted, migrate into tumors and exert their cytotoxicity via NKG2D receptor engagement, allowing their clinical application in a wide range of solid and hematological malignancies (35,36).
CAPRI cell therapy uses the patient’s own peripheral blood monocytes, which present cancer peptides, to prime in vitro naïve T cells into cytotoxic effectors. The CAPRI quartet contains monocytes, DCs, CD4+ and CD8+ T cells. The procedure followed for their generation starts with autologous T cell stimulation with OKT3 and IL-2, which next activate monocytes to display more cancer immunogenic peptides. Subsequent co-culture of these monocytes with unstimulated T cells primes the expansion of CAPRI cells, which upon infusion, exhibit boosted cytotoxicity against tumors. The first results of CAPRI cell therapy in patients with metastatic breast cancer led to prolongation of their survival, compared to non-treated patients (37), whereas favorable results were also shown in non-small cell lung cancer (NSCLC) patients. It should be noted that preparative lymphodepletion by chemotherapy or whole body irradiation usually renders the recipient prone to all of the aforementioned ACT approaches, enhancing adoptively transferred cell persistence and their in vivo anti-tumor effectiveness (38,39).
T cells constitute a further option for cancer immunotherapy, as they can be genetically engineered to express TCRs with high avidity for specific tumor antigens (Table 2). TCR genes of variable origin (e.g., human or from humanized mice) are cloned into viral vectors and used to transduce autologous T cells from patients. The first clinical trials in melanoma patients were promising (40).
Full table
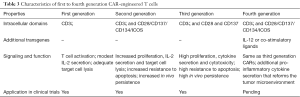
CARs, initially constructed in 1989, are surface proteins that combine the single chain variable fragments (scFv) of an antibody recognizing a tumor antigen with intracellular T cell signaling domains (3). First generation CARs comprised a scFv joined to the CD3ζ chain (41) (Table 3). Second and third generation CARs contained additional co-stimulatory domains such as CD28 and/or CD137, which improved cytokine production by and in vivo persistence of infused CAR-modified T cells, respectively. Recently developed T cells redirected for universal cytokine-mediated killing (TRUCKs/fourth generation CARs) are modified with an inducible IL-12 vector and, upon engagement of the cognate antigen, release IL-12 (42,43). IL-12 sensitizes APCs within the tumor microenvironment and produces a local inflammatory response, improving tumor eradication (44). To bypass T cell modification for a distinct tumor antigen, universal CARs with unlimited antigen adaptability have also been designed. These avidin (45) and anti-fluorescein isothiocyanate (FITC) (46) -bearing CARs bind with high affinity any biotinylated or FITC-labeled tumor antigen-specific mAb and exhibit potent anti-tumor activity.
Full table
First generation CARs used in early clinical trials showed no objective clinical responses. However, clinical trials on hematological malignancies using second or third generation CARs demonstrated notable responses (47). CAR T-meso in patients with mesothelin-expressing tumors and CAR T cells secreting IL-12 for recurrent ovarian cancer are among the most promising constructs (48). Lymphodepletion prior CAR infusion (38,39), cytokine supplementation, integration of multiple co-stimulatory intracellular domains (49) and expression of chemokines and their receptors (50) enhanced persistence and homing of CAR T cells to the tumor site, and were strongly correlated with treatment outcome (51).
Nevertheless, CAR therapy is accompanied by adverse events (e.g., toxicity due to cytokine storm), imposing the need to regulate uncontrolled or hyper-activation. For this reason, negative regulation approaches, like incorporation of suicide switches (e.g., an inducible caspase-9 gene) in CAR T cells were developed. Unfortunately, such strategies led to complete eradication of the engineered cells from the patients’ circulation (52). In contrast, positive regulation strategies, recently applied, integrate in the CAR construct a domain, which requires both an exogenous user-provided signal (e.g., rapamycin analogs) and the scFv target-antigen for its activation (53,54). The clinical efficacy of such transient CAR-modified T cells needs to be evaluated.
Active cancer immunotherapy
Peptide vaccines can generate effective anti-tumor T cell responses
Anti-cancer vaccines are designed to induce tumor-specific or tumor-reactive immune responses in vivo; the most popular category comprises peptide-based vaccines, usually consisting of immunogenic epitopes from tumor-specific or tumor-associated antigens (TSAs or TAAs, respectively). Most tumor antigens derive from products of mutated oncogenes (e.g., K-RAS, BCR/ABL) or tumor suppressor genes (e.g., p53), oncogenic viruses (e.g., HPV, HBV, EBV), oncofetal proteins (e.g., CEA, a-FP), cell-type specific differentiation proteins (e.g., PSA, Melan-A/Mart-1), overexpressed or aberrantly expressed self-proteins (e.g., HER-2/neu), or altered glycolipids and glycoproteins (e.g., MUC-1, CA-125, GM2). TAAs also include cancer-testis (CT) antigens, whose normal expression is restricted to male germ cells in the testis (55). In fact, the first tumor antigens cloned were the melanoma-associated antigen 1 (MAGE-1) and the New York esophageal squamous cell cancer-1 (NY-ESO-1), both classified as CT antigens (56).
Initial clinical trials with TSA- or TAA-derived peptide vaccines used as monotherapy, showed limited effectiveness due to the narrow spectrum of immune responses induced in vivo and the limitation of MHC-restriction (57). Single and multiple peptide vaccines restricted to selected patients expressing the appropriate MHC alleles were followed by vaccines comprising of cytotoxic T lymphocyte (CTL) and helper T (Th) cell epitopes, albeit efficacy was not much improved. The new generation of anti-cancer peptide vaccines consists of multi-peptide cocktails, synthetic long or hybrid peptides, which include both CTL and Th cell epitopes. These are administered in combination with other therapies and are used for the treatment of various types of cancer (e.g., colorectal, lung, pancreatic, gastric, prostate and breast). Specifically in breast cancer, vaccines containing epitopes of HER2/neu, MUC1 and CEA have been tested in phase I-III clinical trials, showing promising results (58). Personalized peptide vaccination (PPV) is also gaining ground, based on the concept of boosting pre-existing host immunity. In the most recent randomized phase II clinical trial, PPV combined with metronomic low-dose cyclophosphamide in patients with metastatic castrate-resistant prostate cancer prolonged OS of those patients who responded immunologically after vaccination, i.e., mounted peptide-specific humoral and CTL responses (59).
Dendritic cells (DCs) are ideal vehicles for anti-cancer vaccine delivery
DCs have been characterized as nature’s adjuvant because of their high potency to initiate and support immune responses (60). Given their potential to stimulate both adaptive and innate anti-tumor immunity, DCs have been used in recent years as “vehicles” of cancer vaccines. Traditionally, two DC-based vaccination approaches have been widely applied, direct targeting of antigens to DC receptors in vivo and ex vivo generation of antigen-loaded DCs. To improve DC-based vaccine efficacy and given the growing understanding of DC biology, research focuses on exploiting the competence of different DC subsets, optimizing ex vivo DC maturation and manipulating co-stimulatory molecule expression (61).
The most widely used DC subset in the clinic is differentiated from peripheral blood monocytes ex vivo cultured with recombinant granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-4 (mDCs). Although proven clinically beneficial, there are both advantages and disadvantages associated with their use. For example, mDCs are easily manipulated before administration, can be loaded with any tumor antigen and optimally activated with a plethora of adjuvants. Nevertheless, ex vivo production of mDCs is labor intensive and costly, and limited numbers thereof are often available. Thus, an attractive alternative is to specifically target DCs in vivo, i.e., load them with the appropriate tumor antigens and activate them to produce pro-inflammatory cytokines. Although in this case, less control over quality and magnitude of induced responses is offered, a number of new molecules are pre-clinically tested, e.g., immune stimulating complexes (referred to as ISCOMs) (62).
The safety, as well as the ability of DC-based vaccines to activate tumor antigen-specific CD4+ and CD8+ T cells in vivo has been thoroughly tested in phase I-III trials for more than 10 years. The first and only, for now, DC-based anti-cancer vaccine that earned FDA approval is Sipuleucel-T (Provenge, Dendreon Corporation) for the treatment of metastatic asymptomatic hormone-refractory prostate cancer. For this, autologous monocytes are harvested from the patient, pulsed ex vivo with a fusion protein of prostatic acid phosphatase and GM-CSF, and then infused back into the patient (63). Moreover, phase II/III clinical trials using similar approaches in patients with melanoma, glioma and glioblastoma, ovarian cancer, RCC and multiple myeloma induced robust responses and improved clinical outcome (64).
A relatively new and promising approach involves the fusion of patient’s DCs with autologous tumor cells, based on the concept that DC/tumor hybridomas can deliver, process, and subsequently present the entire array of patient-specific TAAs, including those yet unidentified. In preclinical models, DC/tumor vaccines resulted in eradication of established tumors. In clinical trials, DC/tumor hybrids were well tolerated, but limited responses were observed in patients with advanced tumors. Current exploitation of means to render each hybrid component more immunogenic before fusion, e.g., by pre-activating DCs with TLR agonists and by pre-treating cancer cells with ethanol so as to express abundant danger signals, is expected to improve their therapeutic potential (65).
From autologous to allogeneic whole cell vaccines
Autologous tumor cells are an apparent source of TAAs for PPV, since, by definition, they encompass all relevant candidate TAAs. Allogeneic tumor cells also represent a good source of TAAs, as in vitro cultured immortalized cell lines: (I) are a limitless source of TAAs; (II) allow for large-scale production of allogeneic vaccines; and (III) provide well-defined batches for use in a wide range of patients, eliminating variability in the composition of the vaccine, facilitating comparison of the clinical outcome and being cost-effective. Allogeneic whole cell vaccines comprise irradiated whole tumor cells that, prior to irradiation, are transfected to produce cytokines or express co-stimulatory molecules, thereby presenting enhanced immunogenicity. Upon administration to patients, the inactivated tumor cells do not proliferate, but express and optimally present a wide range of TAAs to T cells, ideally orchestrating an anti-tumor reactive immune response (66).
A prominent category of whole cell anti-cancer vaccines are GM-CSF gene-transduced tumor cells, which are generally referred to as GVAX. GVAX vaccines have already proven active against a wide range of cancers, including prostate, NSCLC, RCC, pancreatic and melanoma (67). The secreted cytokine GM-CSF acts locally in a paracrine manner, recruits and activates APCs and promotes uptake of shed tumor antigens from irradiated cells for cross-presentation. The most studied GVAX is prostate GVAX, which comprises two GM-CSF gene-transfected irradiated prostate cancer cell lines, PC3 and LNCaP. Although a phase III clinical trial with prostate GVAX was terminated due to increased mortality, pancreatic GVAX is widely tested in phase III trials, especially in combination with checkpoint inhibitors (68).
Checkpoint inhibitors release the “brakes” of the immune system
It is known that cancer cells can be destroyed through processes of cellular immunity, during which T cells recognize and respond to tumor antigens exposed on the surface of APCs. However, optimal T cell activation against the target antigen initiated by the interaction of the MHC molecule-tumor peptide with the TCR, else termed signal 1, should be complemented by the interaction of co-stimulatory molecules on T cells (CD28) and APCs (B7-1/CD80 and B7-2/CD86), i.e., signal 2 (69). Activated T cells also express CTLA-4, a negative regulator of T cell activation that competes for binding to B7, thus counteracting the positive CD28-mediated signals (70).
Another described T cell co-inhibitory pathway involves programmed cell death protein 1 (PD-1) and its ligands PD-L1/L2, the engagement of which leads to decreased T cell proliferation and cytotoxicity, and increased T cell susceptibility to apoptosis (71). Under physiological conditions, the PD-1/PD-L1/2 pathway prevents excessive effector activities by T cells, controls tissue damage during inflammation and prevents the development of autoimmunity by promoting tolerance to self-antigens (72,73). In cancer, engagement of either of the two molecules, leads to T cell exhaustion and down-regulation of the anti-tumor response (74). In other words, tumor cells exploit the PD-1/PD-L interaction as a protective mechanism to “shut down” the generated anti-tumor immune response.
Tumor-induced down-regulation of T cell function can be reversed by using immune checkpoint molecules/inhibitors that block CTLA-4- and/or PD-1-mediated signaling cascades, consequently preserving and maintaining T cell activation within the tumor microenvironment (75). Additionally, it has been recently reported that inhibiting the CTLA-4 pathway leads to depletion of suppressive Tregs and blocking of the PD-1 pathway revives the functionality of exhausted T cells (76). Most importantly, simultaneous blockage of both pathways significantly amplifies the anti-tumor reactivity of T cells (77). Although following CTLA-4 and PD-1, a plethora of immune checkpoints have been identified and numerous clinical trials explored the clinical utility of their blockade, to date only three immune checkpoint inhibitor mAbs have received FDA approval for the treatment of cancer, the CTLA-4 inhibitor ipilimumab (in 2011), and the PD-1 blockers nivolumab and pembrolizumab (both in 2014; Figure 2).
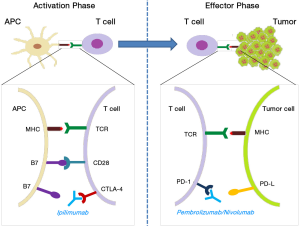
Ipilimumab
Ipilimumab (Yervoy, Bristol-Myers Squibb) is a fully human IgG1 mAb that blocks the interaction of CTLA-4 and B7-1/2. As of February 2016, ipilimumab is approved for treating unresectable or metastatic melanoma, where it demonstrated significant survival advantage (78,79). Currently, 120 open clinical studies are or will be recruiting patients with various types of cancer, who will receive ipilimumab as monotherapy (e.g., for recurrent platinum-sensitive ovarian cancer; NCT01611558) or as a combinatorial treatment (e.g., with chemoradiation for locally advanced cervical cancer; NCT01711515).
Pembrolizumab
Pembrolizumab (Keytruda, Merck) is a humanized IgG4 mAb that targets PD-1, thus disrupting its inhibitory interaction with PD-L1/2. As of February 2016, pembrolizumab is approved for the treatment of advanced melanoma progressed on ipilimumab, and BRAF mutant melanoma progressed on a BRAF inhibitor (e.g., dabrafenib, vemurafenib). It is also approved for treating metastatic PD-L1+ NSCLC, after progression on platinum-containing chemotherapy and/or on EGFR/ALK-targeted medication (e.g., erlotinib, gefitinib, ceritinib) for tumors bearing EGFR or ALK mutations (80-82).
Nivolumab
Nivolumab (Opdivo, Bristol-Myers Squibb) is a fully human IgG4 anti-PD-1 mAb with the same mode of action as pembrolizumab, but with 10-fold reduced affinity for PD-1 compared to pembrolizumab. As of February 2016, it is approved for the treatment of metastatic melanoma progressed on ipilimumab and BRAF-mutated melanoma progressed on a BRAF inhibitor (e.g., vemurafenib) (79,83). Recently, it was also approved for the treatment of patients with metastatic squamous NSCLC with progressive disease on or after platinum-based chemotherapy (84). Nivolumab has demonstrated promising anti-tumor activity in kidney cancer, showing a survival advantage and durable responses regardless of tumor PD-L1 expression levels (85,86). Most importantly, nivolumab displayed less adverse events and lower toxicity, compared to ipilimumab (87).
Due to the high clinical efficacy of immune checkpoint inhibitors, the evaluation of their combinatorial synergistic oncotherapeutic effects is actively being investigated in clinical trials. The first phase I study evaluated the combination ipilimumab plus nivolumab in patients with advanced stage III/IV melanoma. Patients who progressed on ipilimumab achieved positive anti-tumor responses after subsequent treatment with nivolumab, suggesting that sequential administration of immune checkpoint inhibitors can be used to restore anti-tumor immunity in patients progressing upon treatment with one inhibitor (88). Among others, a phase III trial (NCT01844505) evaluating the same combination is currently ongoing for patients with previously untreated unresectable or metastatic melanoma.
Taken altogether, licensing of immune checkpoint inhibitors has yielded impressive therapeutic results, thereby opening new frontiers for their use in other cancer types, including brain tumors, head and neck squamous carcinoma, pancreatic adenocarcinoma, bladder urothelial tumors, gastric and breast cancer.
Oncolytic viruses directly lyse tumors and systemically stimulate anti-tumor immune responses
Lytic viruses, by definition, replicate inside a host cell, which is subsequently destroyed. Oncolytic viruses are innocuous viral strains that selectively target and kill tumor cells, but, as reportedly shown, also induce tumor-specific cell-mediated immunity. Specifically, viral replication in cancer cells, followed by cancer cell lysis, results in the release of more viruses, as well as of tumor antigens (89). Consequently, antigen uptake by APCs indirectly promotes a systemic T cell immune response (90,91). Genetic engineering of oncolytic viruses may further enhance their oncolytic properties and redirect them exclusively in the tumor vicinity (92).
The most prominent oncolytic virus-based therapy, Talimogene laherparepvec (T-VEC), consists of a GM-CSF-transfected modified form of the herpes simplex virus-1 that infects and lyses only tumor cells, with concurrent GM-CSF production. Following an international randomized phase III trial in melanoma patients (OPTiM), where T-VEC exhibited endurable treatment responses, this oncolytic virus was the first to receive FDA approval for use in melanoma patients with unresectable tumor lesions (93). Oncolytic viruses have also shown therapeutic potential in preclinical models, when combined with other immunotherapeutic modalities, such as ACT (e.g., increased tumor trafficking and cytotoxicity of adoptively transferred T cells loaded with vesicular stomatitis virus) (94,95) or checkpoint inhibitors (e.g., tumor regression after administration of vaccinia strains and anti-CTLA-4) (96). Some issues need further investigation, particularly if oncolytic viruses are administered systemically. These relate to increased toxicity, mainly due to sequestration in various organs; ineffective viral dissemination, due to clearance by macrophages or neutralization by pre-existing Abs and complement; and reduced infiltration in the tumor microenvironment, due to low extravasation and the presence of connective tissue and extracellular matrix barriers (97).
Some novel approaches that can further boost anti-cancer immune responses
New delivery methods facilitate in vivo transfer of therapeutic molecules
Over the last years, a variety of nanocarrier systems have been evaluated, as they comprise an appealing vehicle for highly targeted therapy. Nanomaterials can achieve selective, localized and even simultaneous delivery of multiple immunomodulators, TAAs and drugs to targeted tumor sites or lymphoid tissues (98). Among the most frequently used nanoparticles are polymeric nanocarriers like the FDA approved synthetic biodegradable polylactide, lipid nanocarriers like liposomes and phospholipid micelles, acid-degradable hydrogels, gelatin-based nanocarriers and the most modern metal nanocarriers, like quantum dots, which are additionally used for imaging analysis. For example, gold nanoparticles have been shown to increase the immunostimulatory effect of the CpG oligonucleotide adjuvant when coupled together (99). Other preclinical studies reported that poly-lactic-co-glycolic acid nanoparticles delivering melanoma antigens induced an effective anti-tumor CTL response (100). In general, nanocarriers bypass the problem of inconsistent antigen delivery and uptake and constitute a promising anti-cancer modality for the future.
Exploiting immunogenic cell death in anti-tumor immunity
A novel concept first described in 2013, is that certain cancer treatment modalities, like radiotherapy and chemotherapy with specific drugs (e.g., anthracyclines, oxaliplatin) induce in tumor cells the so-called immunogenic cell death (ICD). ICD is a cell death mode characterized by: (I) excessive and extended lysis and release of tumor cell components, including intracellular danger/alarm signals; (II) increased activation of DCs; (III) high uptake and presentation of tumor antigens by DCs; (IV) cross-priming and expansion of tumor-specific CTLs; and (V) production of tumor-specific mAbs. Chemoradiotherapy can also alter the frequency and function of regulatory immune cells including Tregs, myeloid-derived suppressor cells and tumor-associated macrophages. Important mediators which act as danger signals and, in practice, as in situ vaccines, and are released during ICD include high-mobility group box 1, calreticulin, ATP and fragments of polynucleotides (101).
At present, we still need to understand the molecular and cellular mechanisms underlying ICD. This can eventually lead to the development of (I) algorithms for optimal management of cancer patients, based on ICD induction by the given anti-cancer treatment; and (II) combinatorial treatments of immune modulation therapy with ICD-inducing chemo/radiotherapies. As for the latter, promising results have already been reported from clinical trials in various cancer types (e.g., cervical, head and neck, advanced and metastatic melanoma), evaluating the combination of local irradiation with ipilimumab (102).
Future perspectives
During the last decade, we experienced remarkable progress in cancer therapy. Deeper understanding of cellular and molecular pathways leading to tumor development, escape and spread were clinically translated to novel treatment options. New classes of molecularly targeted drugs emerged and promptly gained approval for use in humans. We managed to reprogram the host’s own immune system components to target and attack cancer. We prolonged survival of patients that previously had no effective treatment options. We improved the quality-of-life of cancer patients and survivors. Most importantly, we noted that immunotherapeutic interventions control tumor growth/relapse for years after treatment completion. Thus, it is of no surprise that in 2015, 16 new drug approvals and 7 expanded indications by the FDA concerned targeted or combinatorial immunotherapy. What we also learned is that the combination of various immunotherapeutics should be elegantly orchestrated on the one hand, to boost anti-cancer responses (PUSH) and on the other, to neutralize or eliminate negative immune regulators (PULL) (Figure 3).
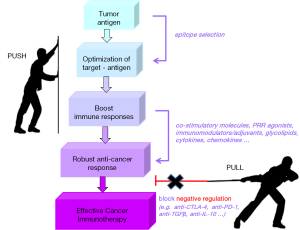
We are on the road to cancer cure. In the years to come, we need to focus on more specific issues, like the development of personalized treatments (e.g., using PPV), the improvement of guided delivery of drugs (e.g., with nanoparticles), the exploitation of the synergy between different cancer treatment modalities (e.g., chemotherapy and checkpoint inhibitors) and the broadening of targeted drug repertoire through the discovery of new targets, new drugs and new molecules targeting multiple molecular pathways (e.g., pan-tyrosine kinase inhibitors). But we also need to invest in the discovery of predictive/prognostic cancer biomarkers that can early enough and reliably guide treatment decisions. Some biomarkers are already available (e.g., PSA, CEA, BRAF V600E mutation), while more are extensively studied (e.g., CTCs, miRNAs). In this way, cancer patients who are likely to benefit from immunotherapeutic interventions will be early on selected, appropriately treated and, hopefully, cured.
Acknowledgements
Funding: The study was supported by European Union FP7 Capacities grant REGPOT-CT-2011-284460, INsPiRE (to OT) and IKY Fellowships of Excellence for Postgraduate Studies in Greece-Siemens (to PS).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007;450:903-7. [Crossref] [PubMed]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 1973;137:1142-62. [Crossref] [PubMed]
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin T cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989;86:10024-8. [Crossref] [PubMed]
- Leach DR, Krummel MF, Allison JP. Enhancement of anti-tumor immunity by CTLA-4 blockade. Science 1996;271:1734-6. [Crossref] [PubMed]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity 2006;24:19-28. [Crossref] [PubMed]
- Racila E, Link BK, Weng WK, et al. A polymorphism in the complement component C1qA correlates with prolonged response following rituximab therapy of follicular lymphoma. Clin Cancer Res 2008;14:6697-703. [Crossref] [PubMed]
- Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res 2010;20:34-50. [Crossref] [PubMed]
- Lundin J, Kimby E, Björkholm M, et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood 2002;100:768-73. [Crossref] [PubMed]
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998;392:86-9. [Crossref] [PubMed]
- Bross PF, Beitz J, Chen G, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res 2001;7:1490-6. [PubMed]
- Alderson RF, Toki BE, Roberge M, et al. Characterization of a CC49-based single-chain fragment beta-lactamase fusion protein for antibody-directed enzyme prodrug therapy (ADEPT). Bioconjug Chem 2006;17:410-8. [Crossref] [PubMed]
- Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005;7:301-11. [Crossref] [PubMed]
- Kim R. Cetuximab and panitumumab: are they interchangeable? Lancet Oncol 2009;10:1140-1. [Crossref] [PubMed]
- Malenfant SJ, Eckmann KR, Barnett CM. Pertuzumab: a new targeted therapy for HER2-positive metastatic breast cancer. Pharmacotherapy 2014;34:60-71. [Crossref] [PubMed]
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumor activity. Nat Rev Cancer 2008;8:579-91. [Crossref] [PubMed]
- Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci 2009;1174:99-106. [Crossref] [PubMed]
- Suryadevara CM, Gedeon PC, Sanchez-Perez L, et al. Are BiTEs the “missing link” in cancer therapy? Oncoimmunology 2015;4:e1008339. [Crossref] [PubMed]
- Belardelli F, Ferrantini M, Proietti E, et al. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev 2002;13:119-34. [Crossref] [PubMed]
- Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine 2004;28:109-23. [Crossref] [PubMed]
- Zhan HL, Gao X, Pu XY, et al. A randomized controlled trial of postoperative tumor lysate-pulsed dendritic cells and cytokine-induced killer cells immunotherapy in patients with localized and locally advanced renal cell carcinoma. Chin Med J (Engl) 2012;125:3771-7. [PubMed]
- Willemen Y, Van den Bergh JM, Lion E, et al. Engineering monocyte-derived dendritic cells to secrete interferon-α enhances their ability to promote adaptive and innate anti-tumor immune effector functions. Cancer Immunol Immunother 2015;64:831-42. [Crossref] [PubMed]
- Akaza H, Tsukamoto T, Fujioka T, et al. Combined immunotherapy with low-dose IL-2 plus IFN-alpha for metastatic renal cell carcinoma: Survival benefit for selected patients with lung metastasis and serum sodium level. Jpn J Clin Oncol 2011;41:1023-30. [Crossref] [PubMed]
- Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 2011;364:2119-27. [Crossref] [PubMed]
- Kilinc MO, Aulakh KS, Nair RE, et al. Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors. J Immunol 2006;177:6962-73. [Crossref] [PubMed]
- Zhao J, Zhao J, Perlman S. Differential effects of IL-12 on Tregs and non-Treg T cells: roles of IFN-γ, IL-2 and IL-2R. PLoS ONE 2012;7:e46241. [Crossref] [PubMed]
- Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 1997;90:2541-8. [PubMed]
- Rook AH, Wood GS, Yoo EK, et al. Interleukin-12 therapy of cutaneous T cell lymphoma induces lesion regression and cytotoxic T cell responses. Blood 1999;94:902-8. [PubMed]
- Younes A, Pro B, Robertson MJ, et al. Phase II clinical trial of interleukin-12 in patients with relapsed and refractory non-Hodgkin’s lymphoma and Hodgkin’s disease. Clin Cancer Res 2004;10:5432-8. [Crossref] [PubMed]
- Little RF, Pluda JM, Wyvill KM, et al. Activity of subcutaneous interleukin-12 in AIDS-related Kaposi sarcoma. Blood 2006;107:4650-7. [Crossref] [PubMed]
- Daud AI, DeConti RC, Andrews S, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol 2008;26:5896-5903. [PubMed]
- Fallon J, Tighe R, Kradjian G, et al. The immunocytokine NHS-IL12 as a potential cancer therapeutic. Oncotarget 2014;5:1869-84. [Crossref] [PubMed]
- Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T cell transfer immunotherapy. Clin Cancer Res 2011;17:4550-7. [Crossref] [PubMed]
- Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344:641-5. [Crossref] [PubMed]
- Wei C, Wang W, Pang W, et al. The CIK cells stimulated with combination of IL-2 and IL-15 provide an improved cytotoxic capacity against human lung adenocarcinoma. Tumour Biol 2014;35:1997-2007. [Crossref] [PubMed]
- Sangiolo D. Cytokine induced killer cells as promising immunotherapy for solid tumors. J Cancer 2011;2:363-8. [Crossref] [PubMed]
- Introna M, Golay J, Rambaldi A. Cytokine induced killer (CIK) cells for the treatment of hematological neoplasms. Immunol Lett 2013;155:27-30. [Crossref] [PubMed]
- Laumbacher B, Gu S, Wank R. Activated monocytes prime naive T cells against autologous cancer: vigorous cancer destruction in vitro and in vivo. Scand J Immunol 2012;75:314-28. [Crossref] [PubMed]
- Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005;202:907-12. [Crossref] [PubMed]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012;12:253-68. [Crossref] [PubMed]
- Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009;114:535-46. [Crossref] [PubMed]
- Eshhar Z, Waks T, Gross G, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T cell receptors. Proc Natl Acad Sci USA 1993;90:720-4. [Crossref] [PubMed]
- Chmielewski M, Kopecky C, Hombach AA, et al. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 2011;71:5697-706. [Crossref] [PubMed]
- Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 2012;119:4133-41. [Crossref] [PubMed]
- Zhang L, Kerkar SP, Yu Z, et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther 2011;19:751-9. [Crossref] [PubMed]
- Urbanska K, Lanitis E, Poussin M, et al. A universal strategy for adoptive immunotherapy of cancer through use of a novel T cell antigen receptor. Cancer Res 2012;72:1844-52. [Crossref] [PubMed]
- Tamada K, Geng D, Sakoda Y, et al. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res 2012;18:6436-45. [Crossref] [PubMed]
- Haji-Fatahaliha M, Hosseini M, Akbarian A, et al. CAR-modified T cell therapy for cancer: an updated review. Artif Cells Nanomed Biotechnol 2015;11:1-11. [Crossref] [PubMed]
- Klebanoff CA, Rosenberg SA, Restifo NP. Prospects for gene-engineered T cell immunotherapy for solid cancers. Nat Med 2016;22:26-36. [Crossref] [PubMed]
- Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA 2009;106:3360-5. [Crossref] [PubMed]
- Di Stasi A, De Angelis B, Rooney CM, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and anti-tumor activity in a Hodgkin tumor model. Blood 2009;113:6392-402. [Crossref] [PubMed]
- Parente-Pereira AC, Burnet J, Ellison D, et al. Trafficking of CAR-engineered human T cells following regional or systemic adoptive transfer in SCID beige mice. J Clin Immunol 2011;31:710-8. [Crossref] [PubMed]
- Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 2011;365:1673-83. [Crossref] [PubMed]
- Wu CY, Roybal KT, Puchner EM, et al. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 2015;350:aab4077. [Crossref] [PubMed]
- Juillerat A, Marechal A, Filhol JM, et al. Design of chimeric antigen receptors with integrated controllable transient functions. Sci Rep 2016;6:18950. [Crossref] [PubMed]
- Pol J, Bloy N, Buqué A, et al. Trial watch: peptide-based anti-cancer vaccines. Oncoimmunology 2015;4:e974411. [Crossref] [PubMed]
- Gnjatic S, Nishikawa H, Jungbluth AA, et al. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res 2006;95:1-30. [Crossref] [PubMed]
- Timmerman JM, Levy R. Cancer vaccines: pessimism in check. Nat Med 2004;10:1279-80. [Crossref] [PubMed]
- Xiao YF, Jie MM, Li BS, et al. Peptide-based treatment: a promising cancer therapy. J Immunol Res 2015;2015:761820.
- Noguchi M, Moriya F, Koga N, et al. A randomized phase II clinical trial of personalized peptide vaccination with metronomic low-dose cyclophosphamide in patients with metastatic castration-resistant prostate cancer. Cancer Immunol Immunother 2016;65:151-60. [Crossref] [PubMed]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245-52. [Crossref] [PubMed]
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012;12:265-77. [Crossref] [PubMed]
- Duewell P, Kisser U, Heckelsmiller K, et al. ISCOMATRIX adjuvant combines immune activation with antigen delivery to dendritic cells in vivo leading to effective cross-priming of CD8+ T cells. J Immunol 2011;187:55-63. [Crossref] [PubMed]
- Obeid J, Hu Y, Slingluff CL Jr. Vaccines, adjuvants, and dendritic cell activators-current status and future challenges. Semin Oncol 2015;42:549-61. [Crossref] [PubMed]
- Ophir E, Bobisse S, Coukos G, et al. Personalized approaches to active immunotherapy in cancer. Biochim Biophys Acta 2016;1865:72-82.
- Koido S, Homma S, Okamoto M, et al. Strategies to improve the immunogenicity of anti-cancer vaccines based on dendritic cell/malignant cell fusions. Oncoimmunology 2013;2:e25994. [Crossref] [PubMed]
- de Gruijl TD, van den Eertwegh AJ, Pinedo HM, et al. Whole-cell cancer vaccination: from autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunol Immunother 2008;57:1569-77. [Crossref] [PubMed]
- Le DT, Pardoll DM, Jaffee EM. Cellular vaccine approaches. Cancer J 2010;16:304-10. [Crossref] [PubMed]
- Amedei A, Niccolai E, Prisco D. Pancreatic cancer: role of the immune system in cancer progression and vaccine-based immunotherapy. Hum Vaccin Immunother 2014;10:3354-68. [Crossref] [PubMed]
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol 2002;2:116-26. [Crossref] [PubMed]
- Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest 2015;125:3377-83. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219-42. [Crossref] [PubMed]
- Bour-Jordan H, Esensten JH, Martinez-Llordella M, et al. Intrinsic and extrinsic control of peripheral T cell tolerance by co-stimulatory molecules of the CD28/B7 family. Immunol Rev 2011;241:180-205. [Crossref] [PubMed]
- Wherry EJ. T cell exhaustion. Nat Immunol 2011;12:492-9. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Turnis ME, Andrews LP, Vignali DA. Inhibitory receptors as targets for cancer immunotherapy. Eur J Immunol 2015;45:1892-905. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [Crossref] [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [Crossref] [PubMed]
- Patnaik A, Kang SP, Rasco D, et al. Phase I study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in patients with advanced solid tumors. Clin Cancer Res 2015;21:4286-93. [Crossref] [PubMed]
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator- choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomized, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. [Crossref] [PubMed]
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomized, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol 2015;33:1430-7. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- O’Sullivan Coyne G, Madan RA, Gulley JL. Nivolumab: promising survival signal coupled with limited toxicity raises expectations. J Clin Oncol 2014;32:986-8. [Crossref] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [Crossref] [PubMed]
- van den Pol AN, Davis JN. Highly attenuated recombinant vesicular stomatitis virus VSV-12'GFP displays immunogenic and oncolytic activity. J Virol 2013;87:1019-34. [Crossref] [PubMed]
- Dranoff G. GM-CSF-secreting melanoma vaccines. Oncogene 2003;22:3188-92. [Crossref] [PubMed]
- Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumor properties. Gene Ther 2003;10:292-303. [Crossref] [PubMed]
- Gil M, Komorowski MP, Seshadri M, et al. CXCL12/CXCR4 blockade by oncolytic virotherapy inhibits ovarian cancer growth by decreasing immunosuppression and targeting cancer-initiating cells. J Immunol 2014;193:5327-37. [Crossref] [PubMed]
- Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015;33:2780-8. [Crossref] [PubMed]
- Qiao J, Kottke T, Willmon C, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med 2008;14:37-44. [Crossref] [PubMed]
- Qiao J, Wang H, Kottke T, et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T cell therapy of tumors. Gene Ther 2008;15:604-16. [Crossref] [PubMed]
- Rojas JJ, Sampath P, Hou W, et al. Defining effective combinations of immune checkpoint blockade and oncolytic virotherapy. Clin Cancer Res 2015;21:5543-51. [Crossref] [PubMed]
- Ferguson MS, Lemoine NR, Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv Virol 2012;2012:805629.
- Irvine DJ, Hanson MC, Rakhra K, et al. Synthetic nanoparticles for vaccines and immunotherapy. Chem Rev 2015;115:11109-46. [Crossref] [PubMed]
- Lin AY, Almeida JP, Bear A, et al. Gold nanoparticle delivery of modified CpG stimulates macrophages and inhibits tumor growth for enhanced immunotherapy. PLoS One 2013;8:e63550. [Crossref] [PubMed]
- Ma W, Chen M, Kaushal S, et al. PLGA nanoparticle-mediated delivery of tumor antigenic peptides elicits effective immune responses. Int J Nanomedicine 2012;7:1475-87. [Crossref] [PubMed]
- Kono K, Mimura K, Kiessling R. Immunogenic tumor cell death induced by chemoradiotherapy: molecular mechanisms and a clinical translation. Cell Death Dis 2013;4:e688. [Crossref] [PubMed]
- Vatner RE, Cooper BT, Vanpouille-Box C, et al. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol 2014;4:325. [Crossref] [PubMed]


